Merging two mobile networks sounds simple until you step inside one. A...
When I arrived at the World Quality Week conference at the Rolls-Royce...
Fiber expansion across North America is opening up new possibilities f...
Telecom deals are easy to announce and hard to operationalize. The hea...
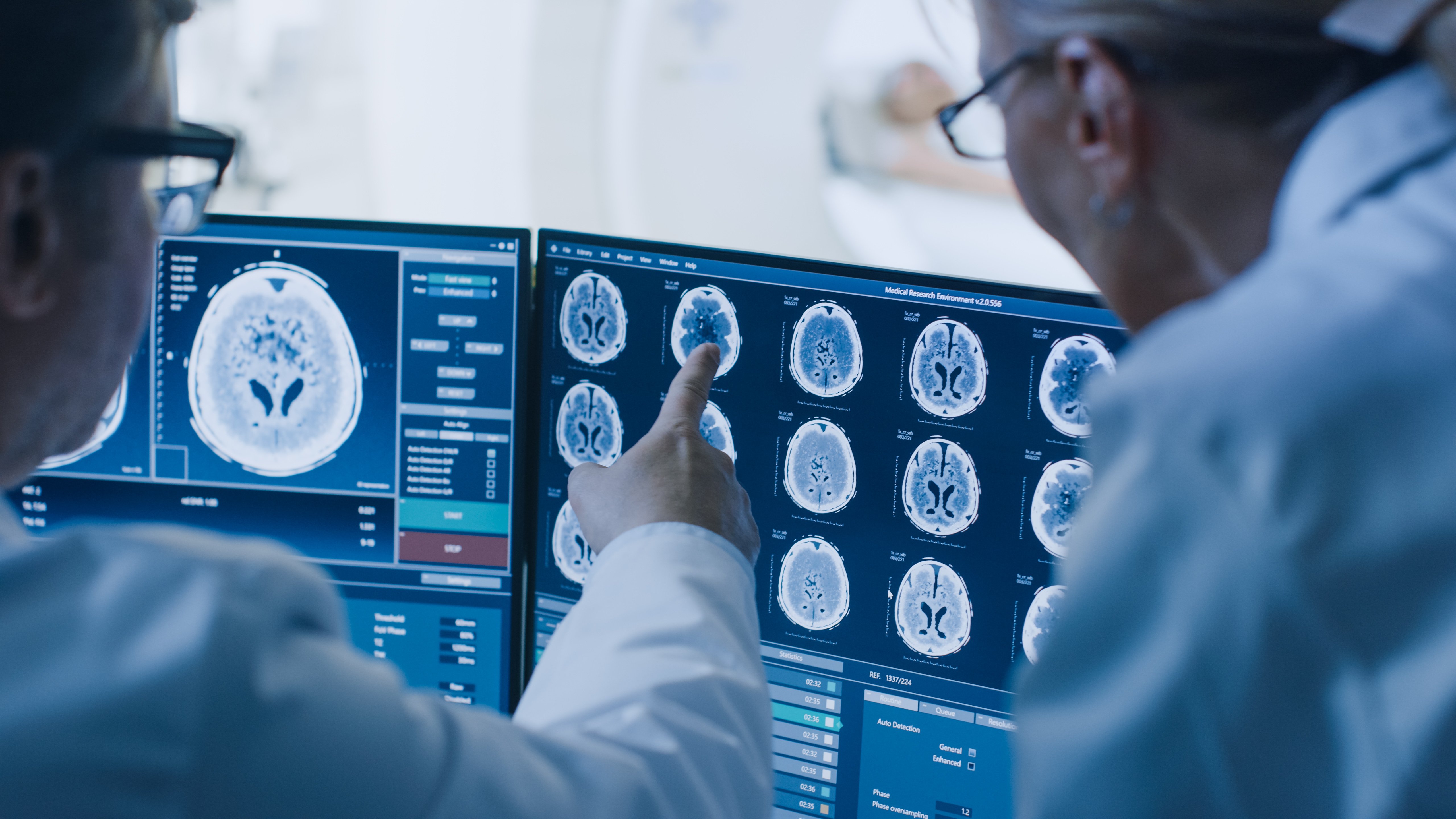
From Experiments to Engineering: What’s Next for Agentic AI in MedTech
“In moments of technological change, impact belongs to those who pair ...
Building the Future of Flight, Together
The aviation industry is entering a defining phase of transformation. ...
RSNA 2025 wasn’t just a conference; it was a marathon. My step tracker...
In today’s constantly evolving telecommunications landscape, networks ...
AI-Led Lifecycle Management: Lessons from Five Industries Shaping the Future of MedTech
Every great product today, whether a car, an aircraft, or a medical de...
In Part 1, I looked at Japan’s CO₂ emissions and how they have changed...
We face a defining moment for engineering. The mid-century climate tar...
In nuclear projects, technical documentation is never just paperwork. ...
Telecom operators are investing billions in 5G infrastructure, but the...
The Urgency of Decarbonizing: Why Nuclear Energy Matters
From Manual to Smart: How Technology is Transforming Utility Pole Inspections
Introduction Across the globe, utility poles form the quiet backbone o...
Urban Planning in the Age of Intelligence Urban planning has always mi...
The Digital side of ESG What if your laptop, your cloud storage, or yo...
Across bustling cities and remote rural landscapes, mobile networks se...
Having moved to Japan from Finland about a year ago, I have been livin...
The Strategic Role of Root Cause Analysis In AIOps In a market defined...
The Esri User Conference 2025 was more than an event—it was a clear si...
Why Energy Sustainability Can’t Be an Afterthought As mobile networks ...
There’s something powerful about being in a room full of people who do...
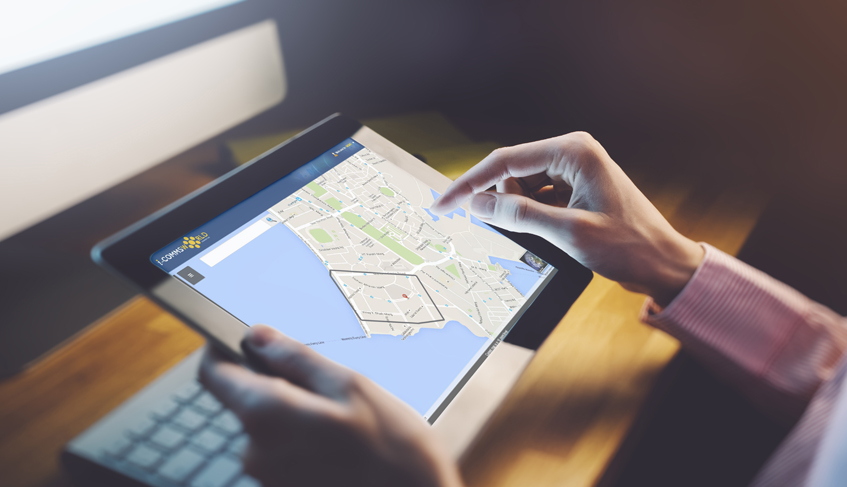
In this concluding installment of our two-part series on Location-Base...
What if utilities stopped treating intelligence as an overlay and made...
Location-Based Services (LBS) have emerged as a powerful enabler for e...
Fiber Connect 2025 delivered what the industry needed—clarity, energy,...
Windows CE, a widely used operating system in embedded systems, has re...
What Drives Our Decisions? Designing a sustainable future that doesn’t...
Enhanced visualization is a technique that transforms a series of 3D D...
Introduction As the demand for high-speed, low-latency Internet contin...
Industries are now undergoing adaptive transformation that will shape ...
GitHub Copilot has rapidly evolved into one of the most influential AI...
From wireless to wireline, Cyient is advancing network intelligence wi...
Software-defined is reshaping how the Industry 4.0 world runs—quietly,...
Transformers, a ground-breaking neural network architecture born from ...
Mobile World Congress (MWC) 2025 has once again solidified its reputat...
In today’s data-driven world, businesses generate vast amounts of raw ...
Every click, every streamed video, and every AI-powered query now dema...
Spatial Digital Twin (SDT) technology is transforming the geospatial i...
The rapid evolution of software development demands faster, more effic...
In today’s hyper-competitive market, businesses must find innovative w...
The evolution of digital twin technology has revolutionized how indust...
As utility companies accelerate digital transformation, smart metering...
Engineering has evolved beyond blueprints and calculations—it’s now a ...
In previous blog posts, we’ve explored the transformative role of Unma...
Unmanned Aerial Vehicles (UAVs), commonly known as drones, have made s...
Clean energy isn’t just a vision for the future—it’s a necessity right...
Digital twin technology is revolutionizing utilities by optimizing ass...
Bird's Eye View (BEV) is revolutionizing industries like autonomous dr...
The global transition to smart grids, powered by digital technologies,...
The advent of the fifth generation of mobile communications (5G) is pa...
The Internet of Things (IoT) is revolutionizing utility operations by ...
Artificial Intelligence (AI) has made remarkable strides in recent yea...
In recent years, the adoption of drones, or UAVs, has played a crucial...
Optimizing energy grids is imperative to meet the evolving needs of mo...
Cyient, a global engineering and technology solutions company, has par...
As research efforts in Mobile Wireless Networks (MWNs) transition from...
A Novel Approach Toward Low-cost Air to Ground Internet Communications
Although satellite and hybrid Air to Ground (A2G) systems are availabl...
In today's hyper-competitive business environment, digital transformat...
Downtime - A costly nightmare for businesses across all sectors, espec...
Rail infrastructure is a tightly controlled and regulated system where...
Explainable Root Cause Analysis in Mobile Networks
In the dynamic world of Mobile Network Operators (MNOs), meeting Servi...
Optimizing Microservices Performance with Distributed Tracing
In recent years, distributed tracing has emerged as a crucial technolo...
The global railway infrastructure and rolling stock industries are on ...
In today’s rapidly evolving energy landscape, the integration of Advan...
Digital transformation is revolutionizing the mining industry, enablin...
Natural gas utility pipeline monitoring and maintenance involve variou...
RPA (robotic process automation) and GenAI (generative artificial inte...
In today's era of advancing technology, face recognition systems have ...
In today's rapidly evolving industrial landscape, embracing digital tr...
In an era marked by rapid technological advancement and increasing env...
Off-road vehicles increasingly feature Advanced Driver Assistance Syst...
Power distribution is changing. The fact is undeniable and one that in...
In the ever-evolving crucible of IT culture, the narrative has shifted...
Optical fiber-based network deployments have evolved to meet the requi...
In the power sector, geographic information systems (GIS) and advanced...
Efficiently managing power in electrical utilities is a complex endeav...
Today, organizations are increasingly turning to digital twin technolo...
In the dynamic landscape of modern manufacturing, collaboration betwee...
The year 2024 promises to usher dramatic change in the energy sector. ...
In Advanced Driving Assistance (ADAS) or autonomous driving systems, o...
Software is increasingly becoming the determining factor in automotive...
A2023 release of the World Energy Outlook Special Report on the oil an...
Network security is a critical aspect of information technology that e...
This is Part 2 of 7-part series explaining how to analyze USE environm...
In recent times, there has been a significant shift in vehicle propuls...
In an era defined by digital connectivity, the ubiquitous presence of ...
In the swiftly evolving landscape of electrical utilities, staying ahe...
Why You Should Consider Migrating Legacy Software to Modern Technologi...
The power industry is witnessing a surge in the adoption of informatio...
With digital transformation strategies, real-time location intelligenc...
Embedded systems have long been used across industries such as aerospa...
In an era where environmental consciousness intertwines with technolog...
Cyient collaboration in ZEQL’s Sustainable Lime Production
Carbon dioxide emissions (CO2) represent a pressing and urgent concern...
Hyperspectral imaging, a groundbreaking technology initially developed...
This is Part 1 of 7-part series explaining the different scenarios and...
The word “radar” stands for radio detection and ranging. This pre-WW I...
In the dynamic landscape of modern industry, how products are conceive...
Utilities across the globe face major challenges due to aging infrastr...
In today's hyper-connected world, seamless wireless network operations...
Autonomous vehicles. Today, the term is as common as AI, cloud and big...
In today's fast-paced world, energy consumption has transcended tradit...
As the automotive industry races towards a future dominated by electri...
The Society of Automotive Engineers (SAE) defines 6 levels of driving ...
Hydrogen is the latest buzzword and game-changer in the world’s energy...
Recent studies have discovered that North America is facing a pressing...
Water conservation is imperative today, given that freshwater sources ...
In the last few decades, dependency on public and private vehicles for...
Enabling smart operations, cost-efficiency with NOCs
Continuous change is a part of today's digital society. We live in an ...
Automotive security is essential to ensure the safety, privacy, and fu...
In today's ultra-connected world, mobile networks are indispensable to...
In recent years, the manufacturing industry has witnessed a technologi...
Urban Air Mobility (UAM) comprises electric vertical take-off and land...
Mines play a crucial role in the extraction of valuable resources, but...
The measurement of physiological parameters is critical in assessing a...
The quality of our rivers and water bodies is vital to the health and ...
With advances in multi-processors, high-performance memory, and securi...
Electric vehicles are rapidly evolving toward providing an eco-friendl...
Software has become an increasingly crucial part of vehicle functional...
In today’s competitive landscape, comprehensive data strategies have b...
With global temperature rising, and greenhouse gases being emitted at ...
Life Cycle Assessment (LCA), also known as life cycle analysis, is a s...
Cloud-native development has become a popular way to build highly scal...
The steel industry forms the backbone of industrial development in any...
Resource depletion has made industries rethink their strategies and re...
Cyient Empowers The City of Oslo in Its Decarbonization Journey
The fight against climate change has had an active demand; Decarboniza...
For those unfamiliar with the term Heritage Monitoring, it refers to t...
Managed vs. Unmanaged C: Increasing Security and Reliability of Embedd...
In a world where technology is advancing at an unprecedented rate, cyb...
The digital economy is reliant on semiconductors, which form the backb...
Urban air mobility (UAM), a complex and technologically advanced syste...
Hybrid work environments and the increasing demand to access applicati...
Securing Virtual Machines in a Cloud Computing Environment
The COVID-19 pandemic inadvertently expanded the cloud computing marke...
Open radio access network (ORAN) is a concept that has been in develop...
Considering net zero targets, the global energy crisis, and reduced fo...
Creating smart and sustainable solutions has become a key focus area a...
Maintaining good health has become an integral part of our lives, and ...
Sustainability isn’t just another buzzword to attract investors and po...
In 2010, a team of former NASA scientists came together in an attempt ...
Technology is continuously evolving and artificial intelligence and ma...
Open AI’s new chatbot ChatGPT has taken the world by storm. Powered by...
In November 2022, Hawaii’s Mauna Loa volcano began erupting in the Mok...
Cameras, Radars, Lidars, Ultrasonic sensors are the various kinds of s...
Eco-anxiety, climate doom, and existential dread have thrown the world...
One of the significant challenges the oil and gas industry faces today...
Sustainability goals and an effort to move toward a greener world have...
Hardware in the form of ECUs (electronic control units) or vehicle pro...
Mining is a trillion-dollar industry globally and one of the largest c...
SUSTAINABILITY – One of the biggest talking points across industries g...
Agriculture is the backbone of the global economy, and with a growing ...
Engineering, Research, and Development (ER&D) companies have been ...
Recently, I had the opportunity to be a part of IInvenTiv, the first-e...
End of Life for ClickSoftware: Why Migrating to FSL Makes Sense for EOL ClickSoftware Users
When Salesforce acquired ClickSoftware in 2019, the product roadmap ch...
Technologies shaping the development of Off- Highway products - Part 1 of the 'Off-Highway Product Series'
Off-highway equipment is the umbrella term used to describe the machin...
Software-defined innovations will sculpt the future roadmap of the aut...
Cybersecurity is of critical importance in a digital world. When it co...
Impact of Changing Hardware on the Automotive Industry Part 4 of the ‘Intelligent Automotive Series’
The Age of Electric Vehicles (EVs)
The previous decade witnessed the transformation of the consumer elect...
Turning Circular
Events in the last couple of weeks that will significantly impact the ...
The Next Decade: Connected, Intuitive and Sustainable Driven by ever-e...
On February 24, 2022, when Russian tanks rolled into Ukraine, it was n...
The opportunities are tremendous. The impact is tangible. What data ha...
Dynamic case management (DCM) involves automating and coordinating asp...
The global maintenance, repair, and overhaul (MRO) market is valued at...
As we approach Earth Day on April 22nd, it is a good time to revisit t...
The COVID-19 pandemic may have increased our social distance and, with...
Innovative thinking can get us out of unforeseen, major crises like th...
Globalization, the decline in long-term technology investments, and in...
The electric vehicle drivetrain offers new freedom in terms of electri...
Attractive packaging is a key factor in marketing products. While desi...
“Leadership is a journey, not a destination” – Bill George
Workforce our core strength
In conversation with Pradeep Elamanchili (Vice President of Global ASI...
Karthikeyan Natarajan, Executive Director and COO, Cyient, in conversa...
COVID-19 has put greater emphasis than ever before on the importance o...
For years, wearable computer devices were popular only among technolog...
Data is the new oil— the most valuable resource in the age of informat...
Global spending on healthcare is expected to reach $11.5 trillion by 2...
According to a recent McKinsey study, 85% of the executives interviewe...
April 2021 marks a personal milestone: 12 months ago I joined Cyient a...
There is a growing need for Network Operators to rethink how they sche...
One of the key aims of a manufacturing company is to have the fastest ...
At the start of 2020, nobody could have predicted that a pandemic woul...
If the COVID-19 pandemic has an upside, it is this: it has helped demo...
2020 will be recorded in history as the year a virus brought the world...
Businesses today operate in a fast-evolving and a hypercompetitive env...
This year I changed jobs and cities. I also changed perspectives. I di...
2020 has been a year of renaissance at multiple levels. It has ushered...
Network operators can improve service quality by empowering field crew...
To me, a quick look at our Q2 business results and my conversations wi...
New technologies impact the way industries operate and function—some w...
With the recent pandemic, the world has witnessed an extreme event tha...
The marketing function during the pandemic is gaining prominence in un...
Companies the world over are accelerating efforts to maximize the valu...
The COVID-19 pandemic has elevated the visibility and importance of da...
The telecom industry has not only grown at a rapid pace over the past ...
2020 is a year marked by uncertainty and volatility. From a raging glo...
This is not the first someone is writing about the ‘gender diversity c...
From connected cars and remote surgery to automated factory floors, th...
Modern medicine as we know it today is just over three hundred years o...
Utilities today are becoming increasingly data-driven, focusing on sol...
NASSCOM, India’s top trade association for IT and ITES estimates the g...
We have all climbed steps – maybe it was a trek, a historical monument...
Economic growth and the progressive social thread of a nation are dire...
A recent WEF report suggests that companies spent an estimated $1.2 tr...
Improving network planning, operation, asset maintenance, and making i...
Building economic sustainability and self-reliance is the foundation f...
One of the positive effects of Covid-19 has been the overriding need t...
2020 will go down in history as a year of reckoning. The humanitarian ...
The Integrated Product Development Process unlocks value across variou...
The coronavirus pandemic has already impacted more than 20 million peo...
Without a doubt, the COVID-19 pandemic has led to an unprecedented dis...
As businesses pivot and align with the new business dynamics and work ...
The COVID-19 pandemic has caused sudden and unprecedented uncertainty ...
The COVID-19 crisis has led to an unprecedented disruption of the worl...
As the world comes to terms with the coronavirus pandemic, widespread ...
Challenging. Demanding. Volatile. These are some of the words we hear ...
COVID-19 is a black swan event for the global economy with extreme aft...
In this two-part blog series, we discuss how smart factories can stay ...
VUCA (Volatility, Uncertainty, Complexity, Ambiguity), first coined in...
The ongoing COVID-19 pandemic has caused an unprecedented amount of tu...
In this two-part blog series, we discuss how Industry 4.0 tools are be...
On January 30, 2020, the World Health Organization declared COVID-19 o...
The COVID-19 pandemic is by far the most disruptive humanitarian and e...
The number of confirmed cases of COVID-19 has crossed the 2.5 million ...
The world is witnessing the biggest pandemic in the last 100 years. Wh...
The COVID-19 fallout is unprecedented. With more than half of the worl...
As the coronavirus dominates global headlines, it is emerging as an un...
At the time of writing this article, WHO estimates suggest that the CO...
As the COVID-19 impact is felt across the world, ensuring capacity and...
The ongoing COVID-19 pandemic has created unprecedented socio-economic...
Modern healthcare and medicine are not limited to R&D biotechnolog...
Nine Things You Can Learn from the Semiconductor Industry for Direction During the COVID-19 Outbreak
Semiconductors are paramount in our daily life. From sensor chips that...
The COVID-19 pandemic is impacting the global economy, trade, and indu...
As the world gears up to battle COVID-19, the importance of diagnostic...
Even in times of economic stability, staying on top of remote factory ...
The impact of the global coronavirus epidemic is evolving by the minut...
The global medical devices industry operates in one of the most challe...
Grid modernization programs currently in progress across electric util...
Imagine you’re a pilot trying to land a commercial aircraft over 200 f...
Communications Service Providers (CSPs) and enterprises, across the gl...
Getting useful information from your analytics solutions requires vita...
Smart Factories are about more than automation. While automation has i...
It’s often said “data is the new oil.” However, like oil, it often sta...
Equipment manufacturers commonly face the challenge of keeping machine...
Businesses collect substantial volumes of data every day from fielded ...
The new in vitro diagnostic regulations (IVDR) that have been publishe...
The number of connected devices in the Internet of Things (IoT) is exp...
In today's world, connectivity is everywhere. With the rapid expansion...
Consider this scenario: you are a new-age Communications Service Provi...
As companies rush to take advantage of the technology evolution era, t...
Airlines today are considered safer, more efficient, and with a highly...
On March 13, 2019, the Council of the European Union published two dra...
Equipment connectivity is key for any organization looking for actiona...
Continuous improvement is vital within the heavy & industrial equi...
Augmented reality (AR) and virtual reality (VR), often referred to as ...
A recent Forrester report, Internet-of-Things Heat Map 2018[1], noted ...
Due to ever-emerging IoT trends and technologies, Gartner Research pre...
In this revolutionary Industry 4.0 era, business leaders in the indust...
Aviation trade shows and expos have always been a melting pot to discu...
The global aerospace and defense (A&D) business landscape is under...
Digital transformation is upending rail communications. Here’s how pro...
Whether using cars or public transport, people routinely complain of d...
Missed Part 1 of this blog? Read it here.
Data Produced by Power Distribution Networks is Critical, but so is Perfecting Data Quality - Part 1
The electric utility industry is undergoing a major transformation dri...
In part one of this blog series, we discussed the key trends that stoo...
In this two-part blog series, we recap the highlights of one of the A&...
In 2003, when Cyient started its engagement with Bombardier Transporta...
“Sitting on the ice, overlooking the ocean, there was a sense of achie...
In a world where technology is constantly evolving, manufacturers and ...
Telecom service providers in Europe are under increasing pressure from...
From the pages of sci-fi novels and movie scripts, self-driving or aut...
Given the increasing shortage of parking spaces in urban areas, many o...
At the end of the day, the goals are simple: safety and security. – Jo...
With the growth of advanced technology in high-speed trains, automated...
In rolling stock projects, often there is a lack of consensus among ma...
Despite the imperative to digitally transform, telecoms are struggling...
Industries across the board are experiencing a paradigm shift in their...
Communication tower infrastructure used to be a drain on CAPEX, but sm...
The Internet of Things (IoT) is one of the most talked about IT trends...
With the first standard for 5G wireless technology approved by the 3GP...
The communications sector is synonymous with innovation and customer e...
As the Internet of Things (IoT) gets more pervasive, industrial engine...
It is becoming easier than ever to bring your business to the forefron...
The telecom industry saw significant milestones being achieved in 2017...
In business, cost structures require constant attention. Even companie...
Next-gen technology is already embedding itself into our lives. From 5...
For the past few years, the telecom industry has been fighting market ...
Healthcare is moving away from hospital environs to patients’ personal...
Building the Smart Cities of tomorrow is no small feat. The telecoms n...
Many businesses are unable to run well without a dependable, high-qual...
The meteoric growth of digitalization has readied us for smart cities,...
Many businesses are unable to run well without a dependable, high-qual...
In order for telecom providers to start savings costs as they prepare ...
Innovation is an outcome of ingenuity and passion. Just as the iPod an...
Machine-to-machine (M2M) simply refers to the communication between de...
The idea of disaster management may not necessarily have been in the b...
The public is ready for superfast, highly reliable 5G networks, but th...
In the world of telecommunications, rolling out, maintaining, and upgr...
When it comes to telecom infrastructure, having an efficient and adapt...
Outsourcing no longer references an operational method businesses use ...
In the dynamic business landscape today, technology is the foundation ...
Today's audiences want networks that facilitate superfast Internet spe...
At Cyient, we are passionate about building trusted, long-term relatio...
The telecommunications industry has conventionally focused on organic ...
Cities around the world are increasingly relying on information and co...
In today’s data-driven world, communications service providers (CSPs) ...
Cyient Collaborates with Microsoft to Create Mixed Reality Ophthalmic ...
The 2017 Engineering Summit brought together over 300 senior participa...
The meteoric growth of big data and advanced analytics is forcing orga...
Consider this scenario-you are a new-age Communications Service Provid...
The technology landscape is rapidly evolving, and client expectations ...
For most university graduates there is one thing on their mind, and th...
Picture this. You are sitting in your garden, but you are able to draw...
The cable industry is growing at a rapid clip across various segments,...
With more than 250 million mobile users and growing, the American tele...
For decades, IT infrastructures have been organised into multi-layered...
The European telecoms market is suffering from depleting revenue strea...
Operational bottleneck for EPCs
Network upgrades to expand high-speed Internet to hard-to-reach rural ...
It's been a hectic year for the global telecom industry. Unexpected po...
For a global company like Cyient, it's easy to be drawn into the macro...
The digital world has helped us accept the concept of smart cities, an...
The communications sector is synonymous with innovation and customer e...
If you're wondering why Africa has been a constant topic of conversati...
Traditional legacy systems have created challenges for today's telecom...
While the current environment may seem bleak for telecom operators, op...
Behind all the buzzwords and wearables, many people fail to realize th...
This is the second part of this article. You can read the first part h...
Unmanned Aerial Vehicles (UAVs) or drones usually have negative connot...
According to the International Monetary Fund, emerging and developing ...
In a career spanning more than 20 years, I’ve spent most of my time in...
Medical technology products frequently face pressures of changing econ...
During the 1890s, urban population in cities like New York and London ...
I read an interesting article recently about Dr. Sandek Ruit, who work...
Energy prices in Australia have continued to rise to unprecedented lev...
We have a few days to go before Telecom Regulators in India are planni...
The word “Smart City” is one of the most frequently used terms that we...
"The most exciting phrase to hear in science, the one that heralds new...
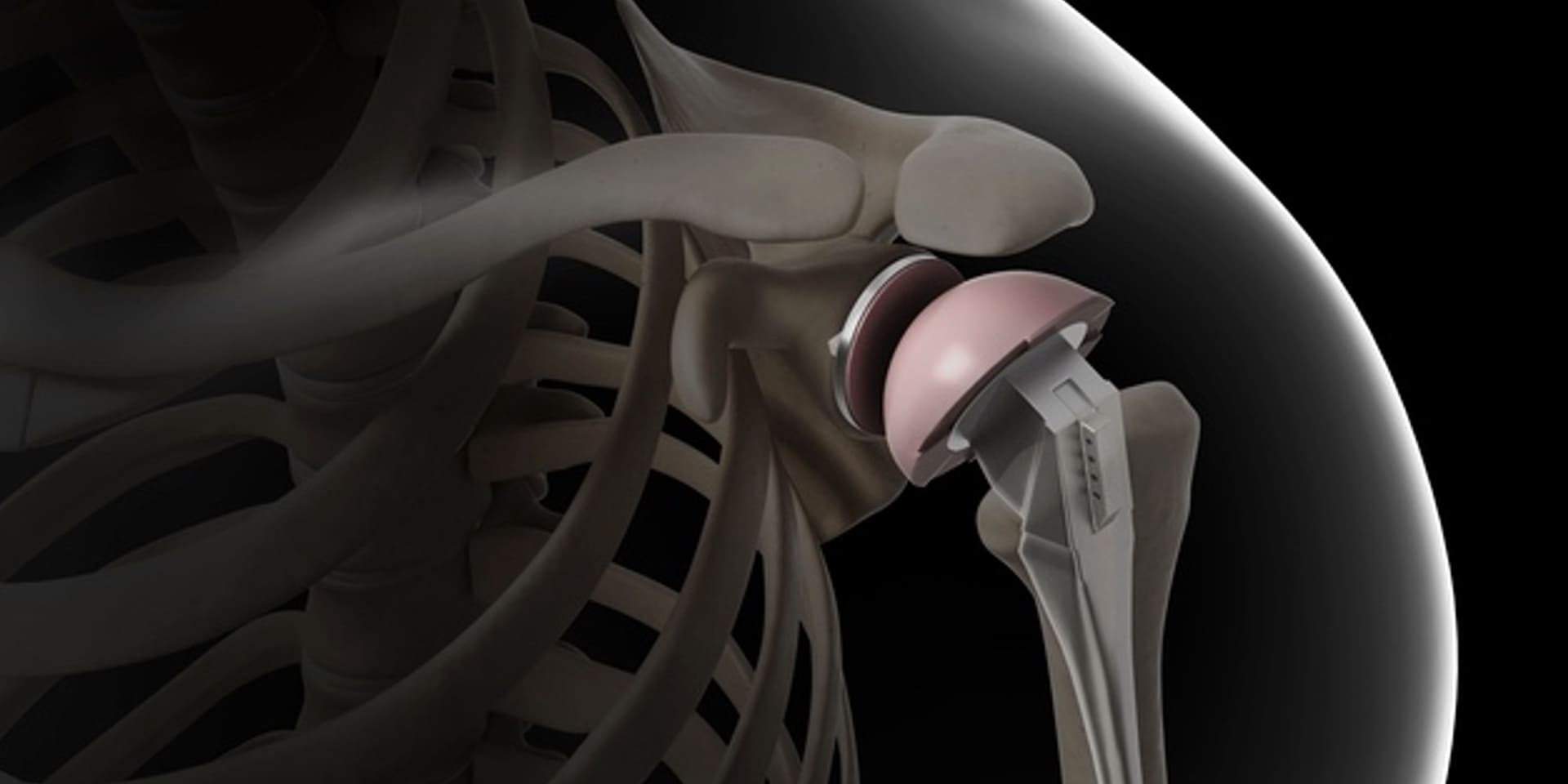
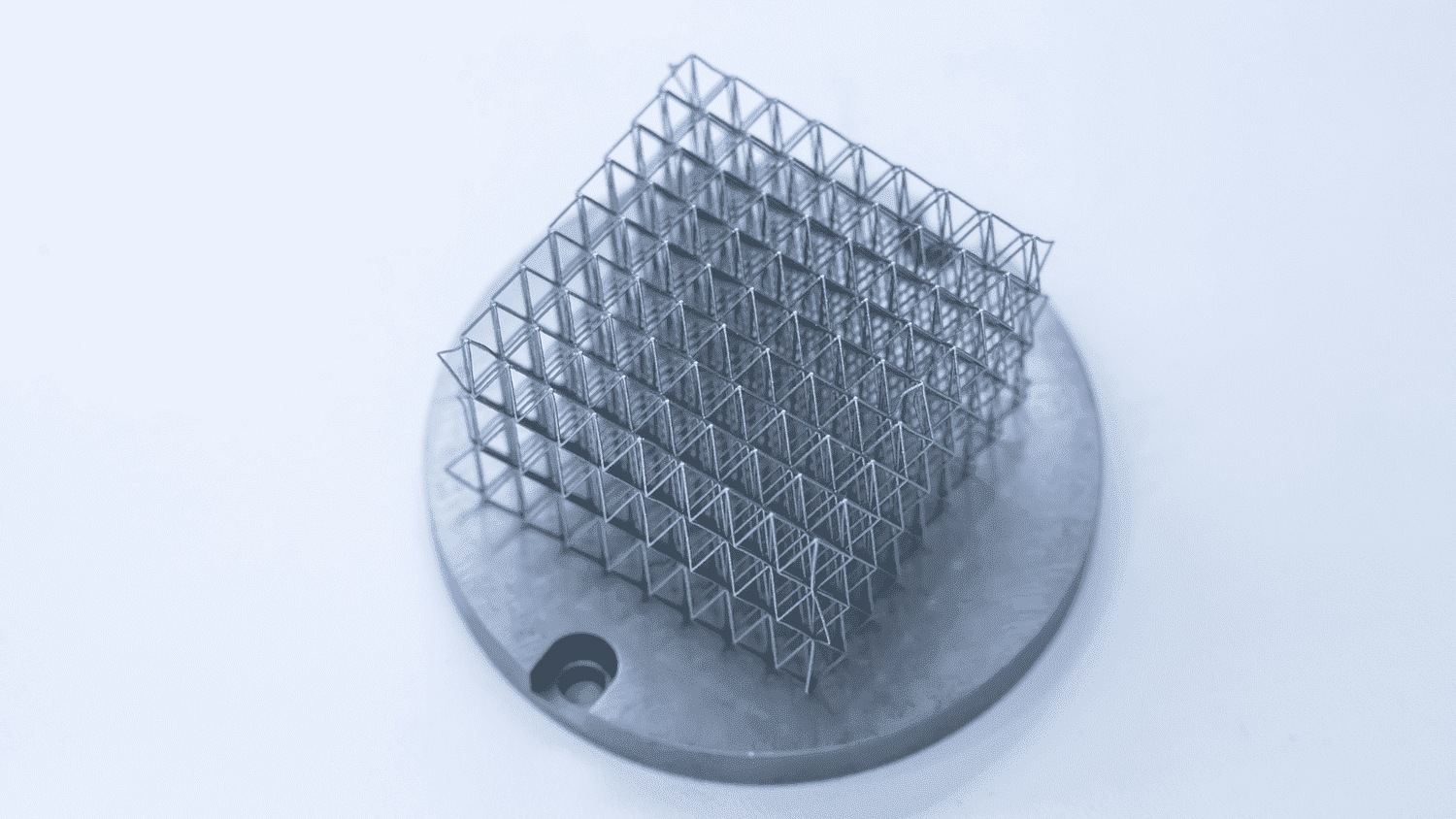
The medical technology industry has been a leader in the use of additi...
Cyient Senior Principal Consultant Harish Lalapeth examines the challe...
Global Medical device manufacturers are more focused than ever on deve...





















































































































.png)











-1.jpg)

.webp)






.webp)




















.webp)

.jpg)






.webp)





.jpg)


























.jpg)

























![[Part 2]-Four Takeaways from Farnborough International Airshow 2018: The Future is Coming... Just Slower than Promised](https://www.cyient.com/hubfs/wonderb.webp)
![[Part 1]-Four Takeaways from Farnborough International Airshow 2018](https://www.cyient.com/hubfs/Imported_Blog_Media/thumb1.jpg)


.webp)











-1.webp)










.webp)











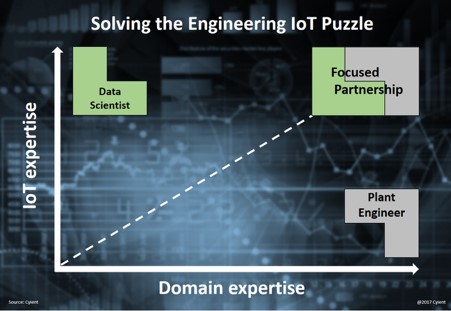




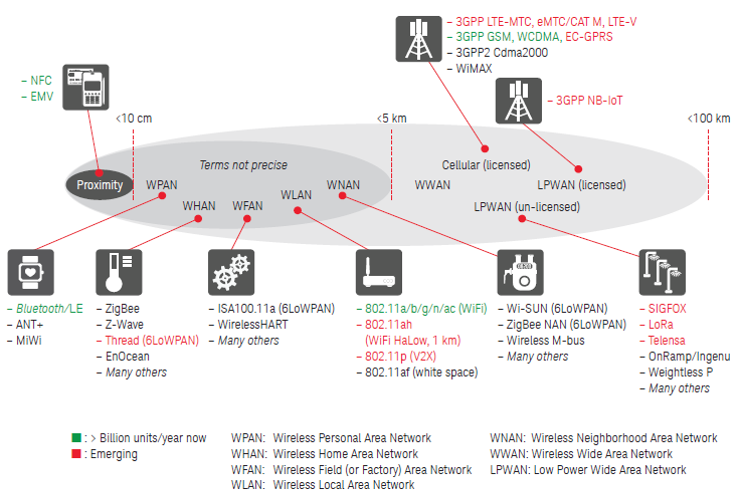











.webp)















Let Us Know What You Thought about this Post.
Put your Comment Below.