Optimizing Medical Device Design: A Comprehensive Guide to User-Centered Engineering
Written by Aboli Manoj Waikar 08 Jan, 2024
This is Part 2 of 7-part series explaining how to analyze USE environment, define USE scenarios, Primary Operating Functions, and derive user interface requirements.
Welcome to our in-depth exploration of user-centered engineering in medical device design. In this seven-part series, we aim to provide a comprehensive understanding of the critical elements involved in creating medical devices that prioritize safety and efficacy. In Part 1, we delved into the significance of human factors in compliance with US FDA regulations and the IEC 62366-1 standard. Now, in Part 2, we will focus on understanding device use, environmental factors, and user interactions.
The Foundation: Human Factors Engineering
As highlighted in Part 1, user-friendly design is not an art but a result of a meticulous engineering process, specifically Human Factors Engineering (HFE). It is imperative to integrate the HFE process with both the product realization and risk management processes to meet regulatory standards. The culmination of this process is captured in the Usability Engineering File, a testament to successful implementation.
While the primary goal of compliance is safety, the broader objectives of task accuracy, completeness, efficiency, and user satisfaction are equally crucial, as outlined in the technical report of IEC 62366-2. Now, let's dive into the core aspects of understanding device use, environmental factors, and user interactions.
Defining Key Terms
Medical Device use is broadly categorized into ‘Reasonably Foreseeable Use’ and ‘Not Reasonably Foreseeable Use’. Scope of usability engineering process is limited to NORMAL Use which is defined as operation, including routine inspection and adjustments by any USER, and stand-by, according to the instructions for use or in accordance with generally accepted practice for those MEDICAL DEVICES provided without instructions for use.
The CORRECT use is defined as NORMAL use without USE ERROR.
The USE ERROR is defined as USER action or lack of USER action while using the medical device that leads to a different result than intended by manufacturer or expected by the USER.
Check below diagram to understand the distinction between them.
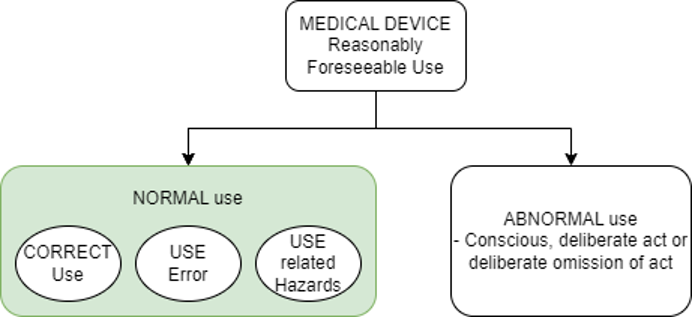
The PRIMARY OPERATING FUNCTION is the function that involves USER interaction that is related to SAFETY of the medical device.
The USER interactions with the medical device according to the accompanying documentation includes following, but not limited to:
- Transport
- Storage
- Installation
- Operation
- Maintenance and repair
- Disposal
In context with explanation of these terms, let’s move towards next step of preparing USE specification.
Preparing Use Specification: Understanding user and use environment
The USER INTERFACE (UI) is the nexus of interaction between the USER and the medical device, encompassing software and physical interfaces such as monitors, displays, alerts, symbols, labels, controls, and accompanying documentation like IFU. Patient or user safety can be ensured when USER INTERACTIONS with medical device are carried out in controlled manner. Regulation mandates that manufacturers shall perform a detailed study of the intended USE environment, USER profile, USER characteristics related to medical device in order to determine safe use of medical device. The output of the study is documented in USE specification, part of USABILITY ENGINEERING FILE. The explicit requirements of this document are described in detail in IEC 62366-1 and also in guidance document published by FDA.
Sections of Use Specification:
- Intended Medical Indication:
- This section serves to articulate the intended USE of the medical device, typically outlined in the 'Customer Requirement Specification' by the Product Manager. This encompasses a broad spectrum, including conditions or diseases targeted for prevention, screening, monitoring, diagnosis, or treatment.
- Intended Patient Population:
- This section, as outlined in the 'Customer Requirement Specification,' may encompass specifics such as age group, weight range, and the health or condition of the patient. It aims to precisely define the demographic parameters within which the device is intended to operate effectively.
- Intended Part of the Body or Type of Tissue:
- Aligned with the 'Customer Requirement Specification,' this section provides details about the specific part of the body or type of tissue with which the medical device is designed to interact. Clarity in this aspect is essential for the device's targeted and effective application.
- Intended USER PROFILE:
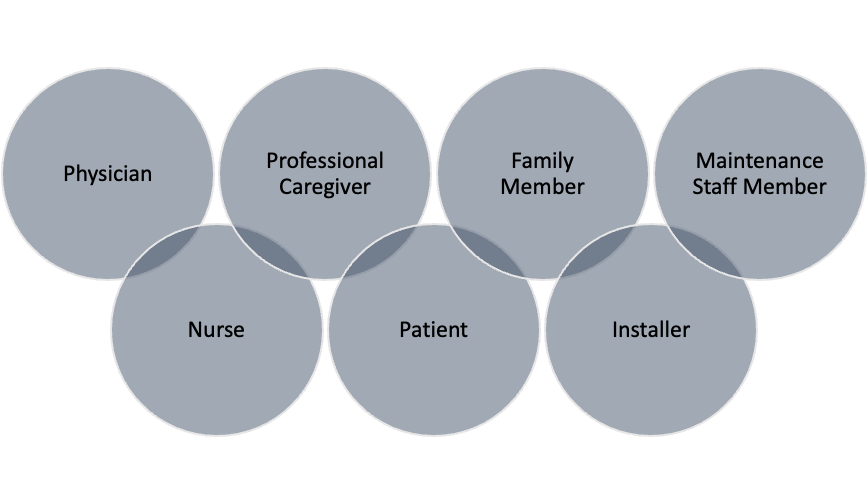
- This critical section elaborates on the intended USERS of the device, with examples illustrated in the figure below.
For the device user, detailed insights into USER characteristics are provided, covering:- Functional capabilities – physical, sensory, and cognitive.
- Experience and knowledge level of the user.
- Behaviors exhibited by the user.
The section also includes information on the level of training required for each User.
- This critical section elaborates on the intended USERS of the device, with examples illustrated in the figure below.
- Intended USE ENVIRONMENT:
- Focused on describing various USE environments, this section outlines settings such as hospitals, surgical suites, homes, emergency scenarios, public spaces, and special environments like emergency transport, mass casualty events, sterile isolation, and hospital intensive care units. The USE environment can vary from simple tasks like registering a patient to complex procedures such as guiding a wire through a catheter on an operation table.
The picture below depicts the complexity of instruments in the vicinity of operation table.
- Focused on describing various USE environments, this section outlines settings such as hospitals, surgical suites, homes, emergency scenarios, public spaces, and special environments like emergency transport, mass casualty events, sterile isolation, and hospital intensive care units. The USE environment can vary from simple tasks like registering a patient to complex procedures such as guiding a wire through a catheter on an operation table.
- Primary Operating Function or Operating Principle:
- This section provides an in-depth exploration of the USER INTERFACE employed for interacting with the medical device. It includes:
- Components and accessories.
- Controls.
- Visual displays.
- Visual, auditory, and tactical feedback.
- Alarms and alerts.
- Logic and sequence of operation.
- Labeling.
- Training.
- This section provides an in-depth exploration of the USER INTERFACE employed for interacting with the medical device. It includes:
The risk management procedure as determined by ISO 14971:2019 demands for including USER INTERFACE characteristics as part of risk analysis. To identify the USER INTERFACE characteristics, the manufacturer shall consider the PRIMARY OPERATING FUNCTIONS, use tools and techniques from USABILITY ENGINEERING PROCESS or refer to a list of questions provided in ISO/TR 24971.
Identifying User Interface Characteristics and Use Errors: Personalizing Design for Safety
The proficiency of a USER in operating a medical device is contingent upon personal characteristics such as physical attributes (size, strength, stamina), sensory abilities, cognitive capabilities, linguistic skills, mental or emotional state, training, and comorbidities. Once the intended user or user group is established, it becomes crucial to evaluate these characteristics that could impact interactions with the device. This consideration is vital as DESIGN INPUT, ensuring that the medical device is robust enough to accommodate variations in USER characteristics. For instance, a mobile X-Ray unit necessitates a specific amount of force to push the unit, and key inputs like the gender and average strength of potential radiological operators are essential in determining the push force limit.
The characteristics of the USE environment constitute another set of factors, defining the various conditions in which the medical device operates, such as hospitals, homes, moving vans, low light conditions, high noise levels, etc. These conditions, which could influence USER INTERACTIONS, must be thoroughly assessed and accommodated in the USER INTERFACE design. This approach aims to make the design more intuitive, safe, and effective, leaving no room for USE ERRORS. For example, the design of an emergency alarm should be crafted to capture the user's attention through visual and/or audible means, even in noisy conditions or during heavy traffic.
Following the analysis of user groups and the environments in which they operate medical devices, potential USE ERRORS can be derived through various methods.
Identification of Known Use-Related Problems:
Understanding known USE ERRORS involves the analysis of clinical data related to the device or devices similar to the one under development. Data sources for this analysis can include customer complaints, discussions with current device users, journal articles, proceedings of professional meetings, newsletters, safety databases like MAUDE, product recalls, safety communications, warning letters, customer feedback surveys, etc. Continuous feedback and improvement can be achieved by regularly monitoring and analyzing clinical data acquired through a post-market surveillance plan for the device.
Cyient offers an internal solution, CyARC, which provides an automated means of collecting information related to safety communication, warning letters, and use-related complaints.
Task Analysis: A Strategic Approach to User Interactions:
Task analysis is an analytical approach employed to assess user interactions with devices. It is utilized to study how users are likely to perform each task, allowing the identification of potential USER ERROR modes for each task. Let's delve into how task analysis can be effectively utilized.
Consider an example of Chest X-ray image acquisition with high level steps as below.
| Sr No | Task |
|---|---|
| 1. | Technician enrolls a patient using image acquisition software. |
| 2. | Technician prepares the patient for X-ray acquisition. |
| 3. | Technician positions the patient against Wall Bucky to take chest x-ray |
| 4. | Technician moves the X-ray tube and adjust the collimator. |
| 5. | Technician selects the type of examination in the software. |
| 6. | Technician reads the acquisition presets kV, mA and adjust the dose as required. |
| 7. | Technician leaves the acquisition room and shoots the X-ray from generator room. |
| 8. | Technician reviews the image acquired. |
| 9. | Technician labels the image as required. |
| 10. | Technician completes the examination and releases the patient if retake is not required. |
Once the user tasks are determined, the next step is to find the answers to below questions for every task to identify potential use errors and associated harms. For example, consider:
Task No. 4. Technician moves the X-ray tube and adjust the collimator.
| Question | Analysis |
|---|---|
| What USE ERRORS user can make while performing the task? | User can make a positioning error, for example, the chest area that needs to be covered is not coinciding with the X-ray area. |
| What could be the circumstances which might cause users to make USE ERRORS? | Technician who has just trained on the system, is not able to understand how much chest area is subjected to X-ray radiation. |
| What is harm associated with USE ERROR? | Positioning error might cause retake of X-ray image acquisition causing extra radiations to patient. |
| How the possibility of occurrence of USE ERROR can be reduced or prevented? | Provision of cross hair laser beam and collimator light in the design representing X-ray acquisition area will guide Technician to align with patient’s chest area. |
| How the severity of potential harm related to the USE ERROR can be reduced or prevented? | Software provisions to restrict on retakes, periodic review of dose reports will prevent retake of images to a certain extent and avoid overdose. |
Task analysis should consider all PRIMARY OPERATING FUNCTIONS and various use scenarios, providing insights into user, environment, and task-related characteristics. A detailed task analysis will help to identify USE ERRORS and potential harms, characteristics related to USER and USE environment. It will also help to identify frequently used functions of the medical device.
Establishing User Interface Specification: Blueprint for Design Success
Utilizing techniques like task analysis helps in establishing USER INTERFACE components and specifications minimizing USE ERRORS, and reducing associated risks. Design considerations such as the size and resolution of the medical monitor, sound decibel limits for alarms, shortcut keys for quick actions, or control placement etc. derived out of this exercise then becomes elements of user interface design and represents testable technical requirements, covering functional as well as the requirements identified as part of risk control measures.
In our next installment, we will explore USE-related hazards, delving deeper into ensuring the safety and efficacy of medical devices through comprehensive design considerations. Stay tuned for the next chapter in our series.
About the Author
Aboli is a Solution Architect, working for Healthcare and Life sciences, CTO Group. An articulate and experienced professional with 18+ years of demonstrative experience in requirements engineering, usability engineering, product risk management, software and hardware testing, verification and validation in Life Sciences and Medical Device domains. Played crucial role in the research, design, development and release of complex Medical Device systems and Clinical Trial related IT applications to deliver secure, compliant, and efficient solutions. Currently, building a solution accelerator "CyARC" with an objective to automate medical device regulatory compliance processes powered by artificial intelligence.
References
- IEC 62366-1:2015+AMD1:2020 CSV
- Applying Human Factors and Usability Engineering to Medical Devices (https://www.fda.gov/media/80481/download)
.png?width=774&height=812&name=Master%20final%201%20(1).png)