- 2026-Feb-17
Understanding the Significance of Human Factors in Medical Devices: Compliance with US FDA Regulation and IEC 62366-1 Standard
Written by Aboli Manoj Waikar Nov 10, 2023 4:29:49 PM
This is Part 1 of 7-part series explaining the different scenarios and use cases of Human Factors in Medical Devices.
Medical devices were once as large as an entire room. However, devices today are smaller than a human palm and have seen a significant shift in form factor. While medical devices are transitioning into modern, user-friendly forms such as smartwatches, compact devices, home-based tests, handheld scanners, and implants, these transformations are also introducing new USE challenges for users. These challenges often lead to USE errors due to consistent changes that these technologies undergo.
Consider these real-life incidents that shed light on the importance of addressing user-related concerns in medical devices:
- A patient experienced an unspecified alarm on an injection infusion pump, resulting in panic and confusion. The patient was unable to troubleshoot the issue or switch to a backup pump, ultimately requiring a 911 emergency call.
- A healthcare worker reported a skin reaction after handling a cassette without wearing gloves.
- During an implant procedure, a physician encountered difficulties with the lead, which could not be implanted as intended. This situation raised concerns about the manufacturing quality of the lead, prompting the use of an alternative lead to complete the procedure. Fortunately, the patient remained stable throughout.
Many medical devices are developed without incorporating Usability Engineering (Human Factors Engineering) Process. As a result, these devices become non-intuitive, challenging to learn, and difficult to use. As healthcare practices evolve, people with the most limited medical expertise, including patients themselves, are using these devices, making usability engineering increasingly crucial.
In an effort to tackle safety complaints related to device usage, developed countries like the United States and the European Union are placing significant emphasis on designing medical devices using usability engineering processes. This approach involves establishing integration points with product realization and risk management procedures. It's important to note that user-friendly design is not simply a matter of artistic flair but rather the result of a systematic engineering process.
The usability engineering process comprises key elements that collectively ensure the development of user-friendly medical devices. These elements include:
- Understanding the User: Gaining deep insights into the users, their needs, and their environment.
- Design and Prototyping: Creating device interfaces that are intuitive, efficient, and user-friendly.
- Evaluation: Conducting thorough assessments to identify and rectify usability issues.
- Iteration and Redesign: Continuously refining the device design based on user feedback and testing.

Regulatory bodies, such as the US FDA and the European Union's Medical Device Regulation (EU MDR), have recognized the importance of the IEC 62366-1:2015 standard and its subsequent amendments. Manufacturers of medical devices are now required to document the history of usability design in a Usability Engineering File, which plays a pivotal role in regulatory submissions, including CE certification and 510(k) approvals.
Components of the Usability Engineering File:
The Usability Engineering File comprises several essential components, each of which plays a crucial role in ensuring the usability and safety of medical devices. These components include:
- User Interface Specification
- Use-Related Hazards
- User Interface Evaluation Plan
- Formative Evaluation
- Summative Evaluation
In this series, we will discuss each of these components in a separate article.
Understanding Human Factors
In the context of medical device usability, human factors, or usability engineering, focuses on the interactions between individuals and the devices they use.
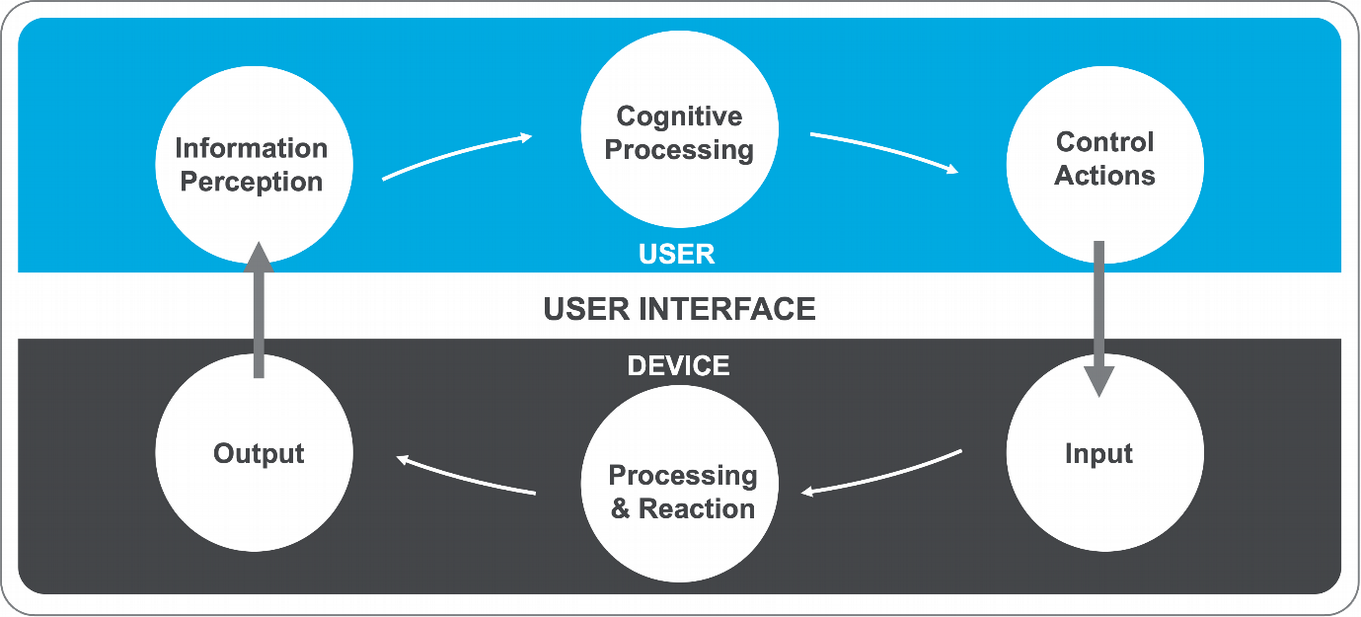
This interaction encompasses how users:
- Perceive Information from the device.
- Interpret the Information and make decisions based on it.
- Manipulate the Device and its controls.
- Receive Input from users.
- Respond to users and provide feedback regarding the effects of their actions.
Human factors/usability engineering is used to design the user-device interface. The user interface includes all components with which users interact while preparing the device for use (e.g., unpacking, set up, calibration), using the device, or performing maintenance (e.g., cleaning, replacing a battery, making repairs).
According to the FDA, the primary objective of human factors/usability engineering in medical devices is to minimize use-related hazards and risks. This ensures that users can safely and effectively use the device. Benefits of applying human factors principles include:
- Safer connections between device components.
- Improved controls and displays interaction.
- Enhanced user understanding of the device's status and operation.
- Better comprehension of a patient's medical condition.
- More effective management of alarm signals.
- Simplified device maintenance and repair.
- Reduced reliance on user manuals.
- Decreased need for user training and retraining.
- Lower risk of use errors and adverse events.
- Reduced risk of product recalls.
Intuitive and error-free design requires intensive user research and references to standards, guidelines and rules of thumb. Let us explore the US FDA regulation, guidance documents and standards available in this context.
The US FDA has incorporated human factors-related requirements into its Quality System Regulations (21 CFR Part §820.30 Design Controls). This integration encompasses various aspects, including user needs assessment, usability verification, and validation. The FDA also provides guidance documents to assist manufacturers in implementing human factors and usability engineering processes effectively.
- Design Input: includes “needs of the user and patient”. Manufacturers are expected to identify user interface related requirements based on frequently used functions and primary operating functions, use case studies, use scenarios, and potential use errors and failures.
- Design Verification: “…design output meets the design input requirements.” Usability verification based on formative evaluation of all prototypes and final versions.
- Design Validation: “...Design validation shall ensure that devices conform to defined user needs and intended uses and shall include testing of production units under actual or simulated use conditions. Design validation shall include software validation and risk analysis, where appropriate.” Usability validation based on summative evaluation techniques to support objective evidence that USE related RESIDUAL RISK has been reduced to acceptable levels.
In addition to the core regulations and standards mentioned above, there are several other important regulatory documents that impact the development and evaluation of medical devices:
- List of Highest Priority Devices for Human Factors Review: The US FDA has issued this draft guidance document to inform medical device manufacturers which device types should have human factors data included in premarket submissions (i.e., for PMA, 510(k)). FDA believes these device types have clear potential for serious harm resulting from use error, and that review of human factors data in premarket submissions will help the FDA evaluate the safety and effectiveness and substantial equivalence of these devices.
- Applying Human Factors and Usability Engineering to Medical Devices: The US FDA has developed this guidance document to assist industry in following appropriate human factors and usability engineering processes to maximize the likelihood that new medical devices will be safe and effective for the intended users, uses, and use environments.
- Human Factors Studies and Related Clinical Study Considerations in Combination Product Design and Development: The US FDA has issued this draft guidance document to assist industry on the underlying principles of human factors (HF) studies during the development of combination products as defined under 21 CFR Part 3. Combination products, as described in 21 CFR Part 3, are comprised of any combination of a drug and a device; a device and a biological product; a biological product and a drug; or a drug, a device, and a biological product.
- Comparative Analyses and Related Comparative Use Human Factors Studies for a Drug-Device Combination Product Submitted in an ANDA: The US FDA has issued this draft guidance document to assist potential applicants who plan to develop and submit an abbreviated new drug application (ANDA) to seek approval of a proposed combination product that includes both a drug constituent part and a delivery device constituent part.
- Content of Human Factors Information in Medical Device Marketing Submissions: The US FDA has developed this draft guidance document. This guidance provides a risk-based framework to guide manufacturers and FDA staff on the human factors information that should be included in a marketing submission to the Center for Devices and Radiological Health (CDRH) to facilitate the efficiency of the FDA review process.
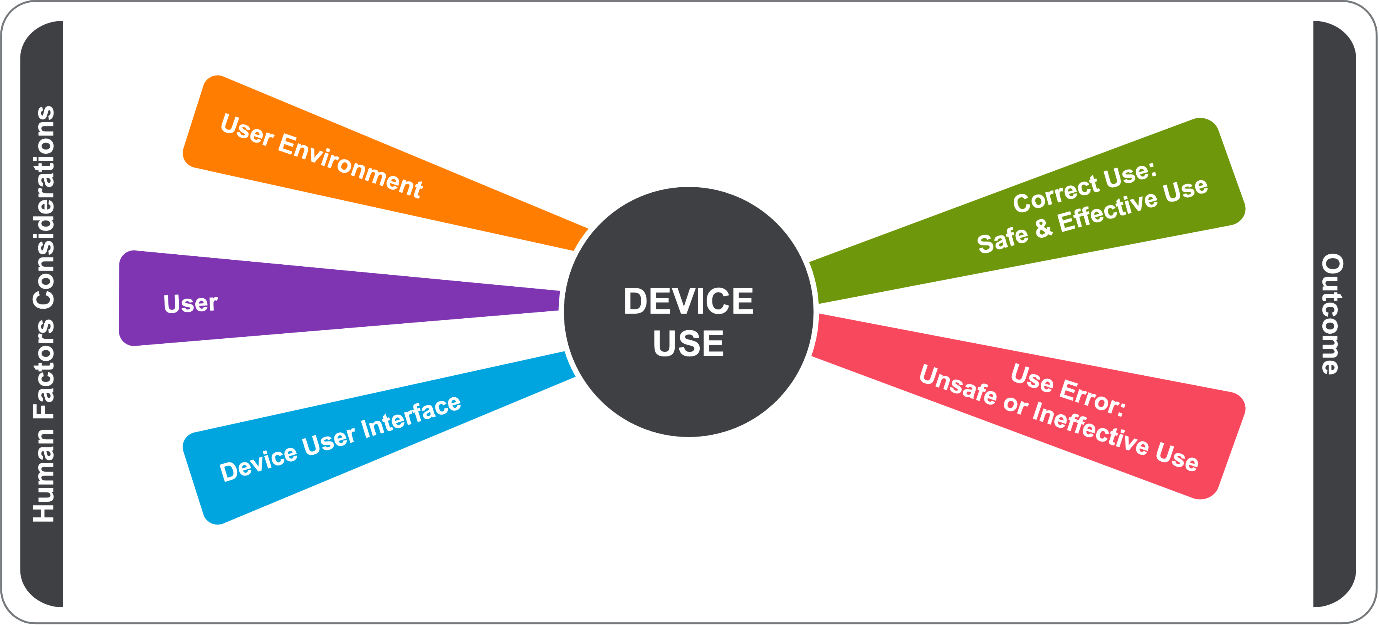
- IEC 62366-1:2015+AMD1:2020 CSV: The US FDA has recognized this standard. This part of IEC 62366 specifies a PROCESS for a MANUFACTURER to analyse, specify, develop and evaluate the USABILITY of a MEDICAL DEVICE as it relates to SAFETY. This USABILITY ENGINEERING (HUMAN FACTORS ENGINEERING) PROCESS permits the MANUFACTURER to assess and mitigate RISKS associated with NORMAL USE, i.e., CORRECT USE and USE ERROR. It can be used to identify but does not assess or mitigate RISKS associated with ABNORMAL USE.
Designing medical devices with human factors considerations is fundamental to ensuring their safety, usability, and effectiveness. In upcoming articles, we will delve deeper into the usability procedure, including the analysis of use environments, definition of use scenarios, identification of frequently used functions, and derivation of user interface requirements, as outlined in the IEC 62366-1:2015 standard.
In the next part of this series, we discuss how to analyze USE environment, define USE scenarios, frequently USED functions and derive user interface requirements. We will also explore the design input document - USER INTERFACE SPECIFICATION as explained in IEC 62366-1:2015 standard.
About the Author
Aboli is a Solution Architect, working for Healthcare and Life sciences, CTO Group. An articulate and experienced professional with 18+ years of demonstrative experience in requirements engineering, usability engineering, product risk management, software and hardware testing, verification and validation in Life Sciences and Medical Device domains. Played crucial role in the research, design, development and release of complex Medical Device systems and Clinical Trial related IT applications to deliver secure, compliant, and efficient solutions. Currently, building a solution accelerator "CyARC" with an objective to automate medical device regulatory compliance processes powered by artificial intelligence.
.png?width=774&height=812&name=Master%20final%201%20(1).png)




