
A Clinical Evaluation Report (CER) documents the clinical evaluation of your medical device. A CER consists of analyzed clinical data that was collected either from a clinical investigation of a medical device or the results of other studies on substantially equivalent devices. The CER demonstrates that the device achieves its intended purpose without exposing users and patients to further risk. Clinical evaluation reports are required for all medical devices in Europe. Medical device manufacturers must submit the CER to the notified body as an attachment to the European CE technical file. The technical file is an essential step to obtaining CE marking for a medical device, required to market medical devices in Europe.
The European Union (EU) is an economic and political union of 28 member states in Europe. It was founded in 1993 with the aim of creating a closer union between the peoples of Europe and has 23 official languages. The CE marking is mandatory for all products sold within the European Union that fall within the scope of CE marking directives.
There are three main directives for medical devices:
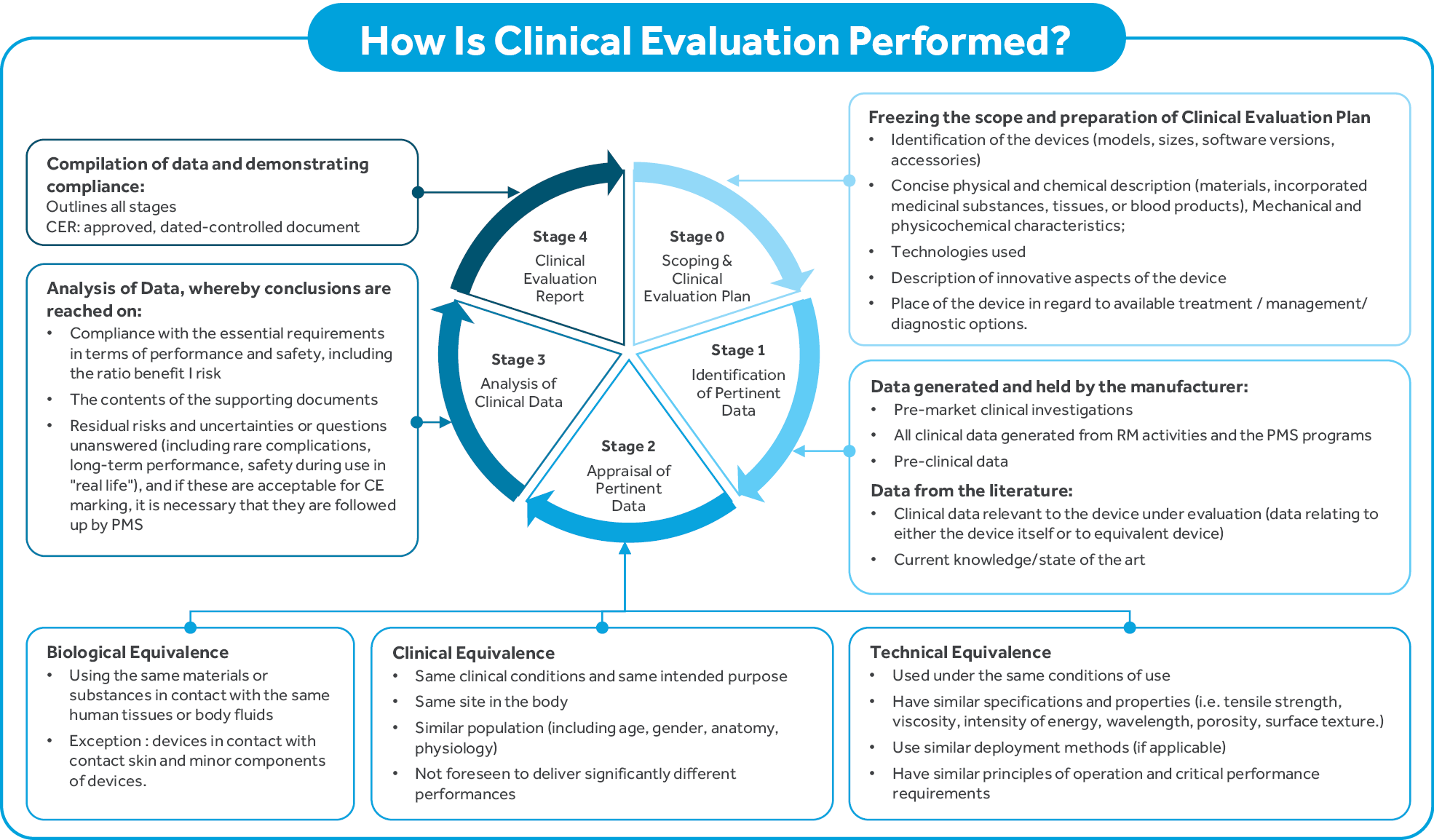
Clinical evaluation is a methodologically sound ongoing procedure to collect, appraise, and analyze clinical data pertaining to a medical device, and to analyze whether there is sufficient clinical evidence to confirm compliance with relevant essential requirements for safety and performance when using the device according to the manufacturer’s instructions for use. Clinical evaluation is conducted throughout the life cycle of a medical device as an ongoing process. Usually, it is first performed during product development to identify data needed for market access. Clinical evaluation is mandatory for initial CE marking and must be actively updated thereafter. Clinical evaluation is necessary and important because it ensures that the evaluation of the device’s safety and performance is based on sufficient clinical evidence throughout the lifetime that the medical device is on the market. This ongoing process enables manufacturers to provide notified bodies and competent authorities with sufficient clinical evidence for demonstration of conformity of the device with the Essential Requirements throughout its lifetime (for example for CE marking, fulfilment of post- market surveillance and reporting requirements, or during surveillance procedures).
Medical device regulatory strategy and phase-wise activity for new product development The regulatory strategy for NPD is carried out in three stages:
Understanding the product and identifying applicable standards as per the intended purpose of the product.
Identifying applicable regulatory requirements and preparation of regulatory assessment report.
Compiling the technical documentation, product registration, and launch.
The clinical evaluation should be conducted by a suitably qualified individual or a team, taking into consideration:


CyARC is a solution offering to accelerate the Regulatory Compliance process for regulated industries such as- Healthcare. It is an automated solution which intends to replace time taking manual and tedious processes and thereby speeding up time-to-market. CyARC’s intelligent framework utilizes NLP techniques and data clustering algorithms. Powered by a rule-based engine CyARC automatically generates pre-populated regulation checklists and templates, device-specific applicability assessments, and gap analysis. It offers the quickest way to provide design input to R&D engineers. CyARC’s digital database is designed to capture information and knowledge. Capable of scaling up, getting trained with user actions, and continuously improving, CyARC’s architecture is flexible and modular. Considering the state of the art, CyARC is one of the most promising solutions to fast-paced product development and sustenance.
A cloud-based solution to accelerate the regulatory compliance process. It helps to search worldwide regulations, and offers a digitized form regulation database for easy search and analysis. The standard module consists of a library of 1500+ international standards such as ISO/IEC/AAMI.
The solution offers a device classification tool, device-specific compliance, and regulatory intelligence services such as regulation assessment, gap assessment, and impact analysis.
Its regulatory watch feature monitors changes in regulations and provides a personalized news feed to users consisting of regulations news, safety communication, and warning letters.

Most medical device companies are racing against time to ensure compliance to EU MDR-217/745 where clinical evaluation is critical. Cyient offers a one-stop solution for helping medical device companies with clinical evaluation for regulatory compliance. Empowered by our Quality Assurance and Regulatory Affairs (QARA) CoE, Cyient has certified professionals across all functions who have the required skill sets and expertise to support medical device companies throughout the clinical evaluation of medical devices.
Abhishek Kumar is an SME in medical device regulatory and quality assurance services. With 12+ years of experience, he has successfully led multiple engagement programs for Europe, US, China, ASEAN markets for NPD and sustenance. Additionally, Abhishek has prepared and implemented the regulatory plan for NPD for 90+ countries by analyzing project feasibility, freezing regulatory requirements, and coordinating with various cross-functional teams.
Cyient (Estd: 1991, NSE: CYIENT) is a consulting-led, industry-centric, global Technology Solutions company. We enable our customers to apply technology imaginatively across their value chain to solve problems that matter. We are committed to designing tomorrow together with our stakeholders and being a culturally inclusive, socially responsible, and environmentally sustainable organization.
For more information, please visit www.cyient.com
Cyient (Estd: 1991, NSE: CYIENT)delivers Intelligent Engineering solutions for Digital, Autonomous and Sustainable Future
© Cyient 2024. All Rights Reserved.