
This whitepaper is aimed at discussing the background, objectives, AMDD structure, potential impact of ASEAN MDD on the ASEAN market, challenges of marketing medical devices in ASEAN market, and how Cyient can help various medical device companies comply and expedite ASEAN MDD compliance.
The Association of Southeast Asian Nations (ASEAN) is a regional grouping that aims to accelerate economic growth, social progress, cultural development, and security cooperation among its 10 members: Brunei, Cambodia, Indonesia, Laos, Malaysia, Myanmar, the Philippines, Singapore, Thailand, and Vietnam. ASEAN was founded on August 8, 1967. Medical device regulatory regimes are under the guidance of the ASEAN Economic Community (AEC). The ASEAN Consultative Committee on Standards and Quality (ACCSQ) was established in 1992 to remove technical trade barriers and facilitate an ASEAN free trade zone (AFTA). It established three harmonized regulatory regimes:
AMDD aims to harmonize standards, technical regulations, and conformity assessment procedures.

The ASEAN Medical Device Directive (AMDD) was published in September 2015. It was signed and accepted by all member states. AMDD is a regional regulatory framework developed by the ASEAN to standardize the regulation of medical devices across member states. Recognizing the increasing importance of medical devices in healthcare delivery, the AMDD aims to ensure the safety, quality, and efficacy of medical devices while facilitating trade and harmonization within the ASEAN market.
Advantages of harmonization: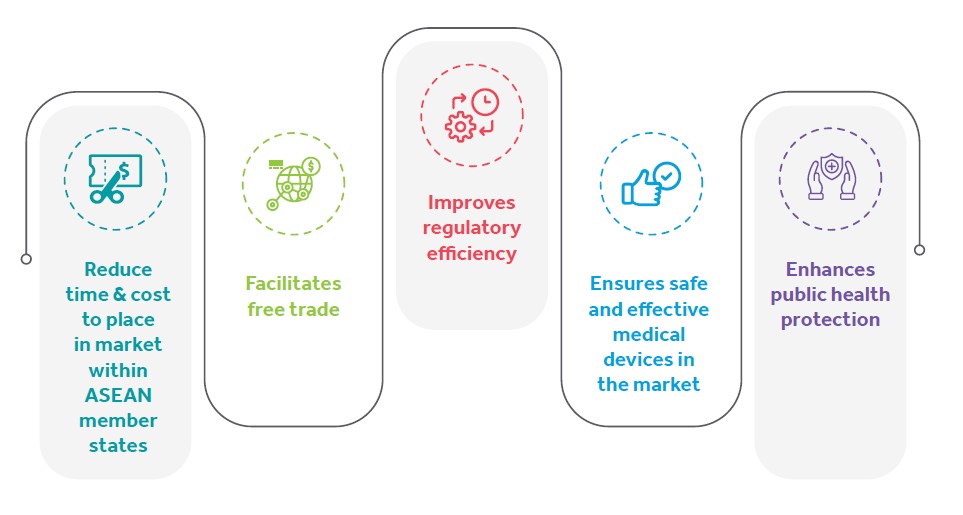

The ASEAN MDD consists of 24 Articles and eight Annexes, as listed below.
|
Article |
Provision |
Description |
|
Article 1 |
General Provisions |
Authorized representative shall register the medical device with and be licensed by regulatory authority of member state. |
|
Article 2 |
Definitions and Scope |
Definitions and scope of the ASEAN MDD. |
|
Article 3 |
Essential Principles of Safety and Performance of Medical Device |
All medical devices shall meet Essential Principles of Safety and Performance (EPSP) of medical devices. |
|
Article 4 |
Classification of Medical Devices |
Medical devices to be classified (A, B, C, D) based on risk level. |
|
Article 5 |
Conformity Assessment of Medical Devices |
Medical devices shall be assessed by the regulatory authority/appointed bodies of member states, unless exempted by member states. |
|
Article 6 |
Registration and Placement on the Market |
Medical devices shall be assessed, registered, and marketed in the member states. Custom-devices are exempt. |
|
Article 7 |
Licensing of Person Responsible for Placing Medical Device on the Markets of Member States |
Member states are responsible for implementing appropriate licensing system. |
|
Article 8 |
Technical Documents for Medical Devices |
Annex 4 – Common Submission Dossier Template (CSDT); Annex 5 – Post Market Alert System (PMAS); and Annex 6 – Declaration of Conformity (DoC). |
|
Article 9 |
Reference to Technical Standards |
Medical devices shall conform to the standards of the ASEAN Medical Device Committee (AMDC) or standards accepted by regulatory authorities of member states. |
|
Article 10 |
Labeling |
Labelling will be done as per Annex 7 or as deemed appropriate by member states. Labeling may be required in national language of member states. |
|
Article 11 |
Medical Device Claims |
Medical Devices shall be subject to regulatory control of Member States. |
|
Article 12 |
Post-Marketing Alert System (PMAS) |
Distribution record, complaint record, Adverse Event Reporting (AER), Field Safety Corrective Action (FSCA). |
|
Article 13 |
Clinical Investigation (CI) |
Member states shall put in appropriate system for conduct of CI, considering Helsinki Declaration. |
|
Article 14 |
Institutional Arrangements |
The AMDC, ACCSQ, ASEAN secretariat, and AMDTC established by AMDC shall coordinate the implementation of the AMDD and shall comprise regulatory authorities of each member state. |
|
Article 15 |
Safeguard Clauses |
Member states shall acknowledge the safety of medical device placed in the market and in their own right terminate the placement of the medical device in case of compromise of safety and noncompliance. |
|
Article 16 |
Confidentiality |
Member states and parties involved in implementing this agreement are bound to observe confidentiality in handling and sharing information. |
|
Article 17 |
Special Cases |
Member states may register or prohibit placement of medical device/refurbished medical device, as they deem appropriate. The AMDD does not limit the authority of member states. |
|
Article 18 |
Implementation |
Member states shall implement the AMDD and shall ensure post market surveillance. |
|
Article 19 |
Revisions, Modifications, and Amendments |
This agreement can be revised, modified or amended, if all member states agree. |
|
Article 20 |
Dispute Settlement |
Mechanism to solve the disputes or disagreement among ASEAN member states. |
|
Article 21 |
Reservations |
Member states shall make no reservation with respect to any of the provisions of AMDD agreement. |
|
Article 22 |
Entry into Force |
January 2015. |
|
Article 23 |
Annexes |
Eight annexes are an integral part of the AMDD. |
|
Article 24 |
Depositary |
AMDD is accepted by all member states. |
|
Annexes |
Provision |
Description |
|
Annex 1 |
Essential Principles of Safety and Performance of Medical Device |
19 Essential Principles of Safety and Performance (EPSP). |
|
Annex 2 |
Risk Classification Rules for Medical Devices Other Than IVD Medical Devices |
16 rules |
|
Annex 3 |
Risk Classification Rules for IVD Medical Devices |
7 rules |
|
Annex 4 |
ASEAN Common Submission Dossier Template (CSDT) |
Based on the Global Harmonization Task Force (GHTF) summary of technical documentation (STeD) format. |
|
Annex 5 |
Post-Marketing Alert System (PMAS) Requirements |
Import/distribution records, complaint records, Adverse Event (AE) reporting criteria and format, Field Safety Corrective Action (FSCA) reporting format. |
|
Annex 6 |
Component Elements of Product Owner's or Physical Manufacturer's DoC |
As per the details requested in this annex, template provided. |
|
Annex 7 |
Labeling Requirements |
Requirements for labels and Instruction for Use (IFU). |
|
Annex 8 |
Clinical Investigation (CI) |
Covers all requirements for CI, EPSP, Post Market Clinical Follow-up (PMCF). |
The Common Submission Dossier Template (CSDT) has reduced the differences in documentation formats that existed in different ASEAN jurisdictions. The adoption of the CSDT in the ASEAN region has minimized the preparation of multiple dossiers, arranged in different formats but with essentially the same contents, for regulatory submission to various regulatory authorities within ASEAN.
Listed below are the contents of the ASEAN CSDT :
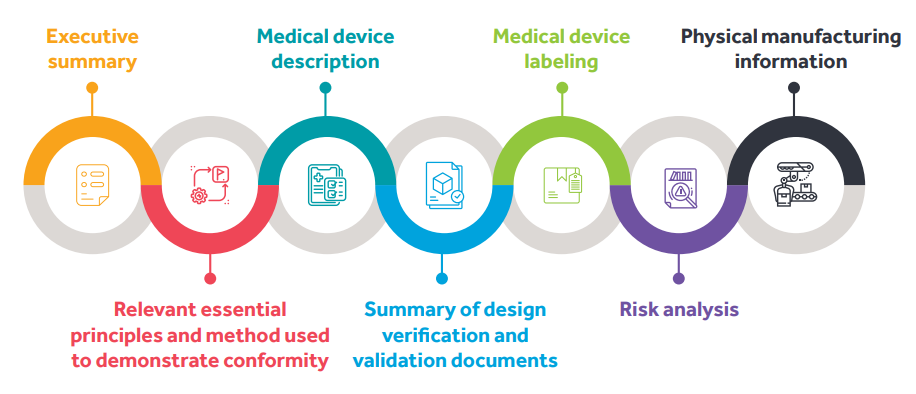
|
EU MDR-2017/745 |
ASEAN MDD |
|
| Year of release | Regulation released in 2017 | Directive released in 2015 |
| Applicable to no. of countries/ regions | European Union, 27 countries | ASEAN, 10 countries |
| Articles in regulation/ directive | 123 Articles | 24 Articles |
| Annexes in regulation/ directive | 16 Annexes | 8 Annexes |
| Harmonized standards | Harmonized standards available | Harmonized standards available in ASEAN Trade Repository (https://atr.asean. org/index.php/standards/) |
| Device classification | Risk-based classification: Class I, II a, II b and III | Risk-based classification: Class A, B, C, D |
| Essential principles checklist | General Safety and Performance Requirements (GSPR) | Essential Principles of Safety and Performance (EPSP) |
| Submission document | Technical documentation | ASEAN Common Submission Dossier (CSD) |
The purpose of embedded software testing is to verify a software's functional and non-functional attributes as well as its bug-free integration with hardware. There are five levels of the testing process for embedded software:
Software unit testing involves testing each unit of software to decide whether it performs as expected. The process involves isolating a section of code and verifying its accuracy during the software development phase. A unit may be a function, a module, an object, a procedure, or a method.
To conduct the test, a framework having information about software codes and real-time operating systems, including details about communication, mechanisms, and interrupts must be developed. A point of control protocol sends and receives messages via message queues. Next, the developer sees the system resources to figure out whether the system can accommodate embedded system execution. Gray box testing is often used for this process.
Integration testing can be further divided into two categories: software integration testing and software/hardware integration testing. A software component is tested in conjunction with a hardware component. You can also use this test to analyze how software interacts with peripheral devices. Embedded tests are always conducted in a real-world environment like the one in which software is developed. Most testers find embedded testing crucial since comprehensive testing can't be conducted under simulated conditions.
During this process, the entire system is contained within a single node. To control and observe, a combination
of communication protocol choice, operating system events, and messages is used. A combination of black and grey box testing is often used for this process.
This is also called acceptance testing. Here, testers ensure that the embedded system and subsystem are perfectly implemented. Analyzing whether the external entity can match the product's functional requirements is the main aim. External entities can be people, devices, or both. In this process, black box testing is often used.
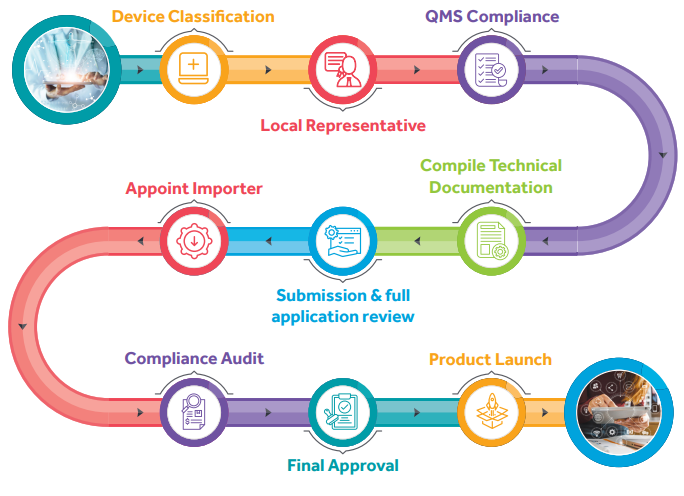
Determine the device classification: Medical devices are classified into four classes based on the risk levels of the devices as per their intended use.
• Class A – Low Risk
• Class B – Low-Moderate Risk
• Class C – Moderate-High Risk
• Class D – High Risk
If applicable, appoint an authorized representative for local market (physical presence in ASEAN member states).
Establish a quality management system as per ISO 13485.
Compile technical documentation as per CSDT.
Submit application for device registration.
If applicable, appoint a registered importer to bring the device into market.
During audit compliance, regulatory authority verifies device classification and performs detailed classification of the submitted application.
Regulatory authority grants final approval.
Launch the product in ASEAN market.
.jpg?width=1000&height=750&name=shutterstock_1917115745%20(1).jpg)
| Market → | USA | Europe | Japan | ASEAN Countries | Non-ASEAN countries | ||||
| General Information | US FDA | EU MDR | PMDA | Thailand | Philippines | Malaysia | Singapore | Vietnam | Taiwan |
| Regulatory Authority | US FDA Address: U.S. Food & Drug Administration 10903 New Hampshire Avenue Silver Spring, MID 20998 |
EU MDD | Pharmaceuticals and Medical Devices Agency (PMDA) | Thailand FDA Address: Ministry of Public Health, Food and Drug Administration, 88/24 Tiwanon Road, Nonthaburi, Thailand |
Philippines FDA Republic of Philippine: Addrass: Civic Drive, Filinvest Corporate Cisy. Alabame. Muntiniupa, City |
Malaysian Medical Device Authority (MDA) Address: Level 6, Prime 9. Medical Devices Granch Prima Avenue I1, Medical Devices Cluster Block 3547, Parsiaran APEC, Health Products Ragulation 63000 Cyberjaya, Selangor, Group; Health Sciences Malaysia |
Health Science Authority (HAS) Medical Devices Branch Prima Avenue I1, Medical Devices Cluster Block 3547, Parsiaran APEC, Health Products Ragulation 63000 Cyberjaya, Selangor, Group; Health Sciences Malaysia Authority: 11 Biopolis Way, #11-03 Helios Singapara 138667 |
Department of Medical Equipments and Health Works (DMEHW) Addess: 138A Giang, Vo, Ba Dinh, Hanoi |
Taiwan Food and Drug Administration (TFDA) Address: Main Office: No 161-2, Kunyang St, Nagang District, Taipei, 115-61, Taiwan (R.O.C) |
| Authorised Representative / Distributor Requirement | Applicable | Applicable | Applicable | Applicable | Applicable | Applicable | Applicable | Applicable | Applicable |
| Regulatory Guideline Requirement | 1. US FDA, 510K guidance document for Industry and FDA staff | EU MDR-2017/745 | Pharmaceuticals and Medical Devices Act (PMD Act) | 1. Medical Device Act B.E. 2531 1998 2. EU MDR 3. US FDA 4. ASEAN MDD |
1. AO2018-002, 2. EU MDR 3. US FDA 4. ASEAN MDD |
1. Medical Device Regulation 2012 1998 2. EU MDR 3. US FDA 4. ASEAN MDD |
1. Medical Device Guidance 2. EU MDR 3. US FDA 4. ASEAN MDD 5. PMD Act |
Decree No. 169/2018/ND-CP (English translation required) | APEC Medical Devices Regulatory |
| Validity of Registration | Medical Device - 5 years | Medical Device - 5 years | Medical Device - does not expire | Medical Device - 5 years Import licence - 1 year |
Medical Device - 5 years | Medical Device - 5 years | Medical Device - do not expire but registration fee shall be paid annually to maintain registration | 1. Type A - unlimited 2. Type B, C & D - 5 years |
Quality System Documentation - 3 years Product licence - 5 years |
| Duration of Registration submission | Class I, II, III - 90 days (510K submission) | Class I, IIa, IIb, III - Technical file submission to notified body which generally takes 40 to 60 days for review | Class I - (General Medical Device) < 1 month Class II (Specified Control Medical Device) 3-5 months, Class II (Specified Control Medical Device) 7-9 months, Class III (Highly Controlled Medical Device) 9-11 months, Class IV (Highly Controlled Medical Device) 13-16 months |
Class I - (High Risk) - 8 to10 month Class II (Moderate Risk) - 6 to 8 months, , Class III (Low Risk) - 10 to 15 days |
Class A - 1 month Class B, C & D - 3 to 6 months |
Class A - 1 to 2 months Class B - 3 to 4 months Class C - 6 to 7 months Class D - 7 to 8 months |
(For full route) Class A - NA Class B - 4 months Class C - 7 months Class D - 10 months Abridged submission takes around 5 months and is cost effective |
1. Type A (Group 1) - 10 days 2. Type B, C & D (Group 2) - (a) 15 days (with national technical regulation) (b) 60 business days without national technical regulation and additional 15 days for supplement documentation needed |
1. Class I - 1 to 2 months 2. Class II & III - 10 to 12 months |
| Language | English | English | Japanese | English & Thai language | English | English | English | English or Vietnamese | English or Chinese |
| Mode of Submission | Digital& Hard copy | Digital | Digital | Digital / Hard copy | Digital | Digital | Digital | Digital | Hard copy |
| Regulatory Complexity | Medium | High | Medium | Medium | Medium | Medium | Medium | Medium | Medium |
The ASEAN Medical Device Directive plays a pivotal role in harmonizing the regulation of medical devices within the ASEAN region. By establishing common standards, classification systems, and conformity assessment procedures, the AMDD enhances patient safety, facilitates trade, and promotes regional integration. While challenges exist in its implementation, the AMDD offers significant opportunities for manufacturers, regulators, and healthcare providers in ASEAN, fostering a more robust and harmonized medical devices market in the region. Cyient offers a one-stop solution for helping medical device companies comply with the ASEAN MDD as well as ASEAN country-specific regulatory requirements. Cyient also offers CyARC (Cyient Accelerated Regulatory Platform) to help accelerate compliance to the ASEAN market. Empowered by our Quality Assurance and Regulatory Affairs (QARA) CoE, Cyient has certified professionals across all functions who have the required skill sets and expertise to support medical device companies in placing their medical devices on ASEAN market.

Abhishek Kumar is an SME in medical device regulatory and quality assurance services. With 12+ years of experience, he has successfully led multiple engagement programs for US, Europe, China, and ASEAN markets for NPD and sustenance. Additionally, Abhishek has prepared and implemented the regulatory plan for NPD for 90+ countries by analyzing project feasibility, freezing regulatory requirements, and coordinating with various cross-functional teams.
Cyient (Estd: 1991, NSE: CYIENT) is a global Engineering and Technology solutions company. We collaborate with our customers to design digital enterprises, build intelligent products and platforms and solve sustainability challenges. We are committed to designing tomorrow together with our stakeholders and being a culturally inclusive, socially responsible, and environmentally sustainable organization.
For more information, please visit www.cyient.com
Cyient (Estd: 1991, NSE: CYIENT)delivers Intelligent Engineering solutions for Digital, Autonomous and Sustainable Future
© Cyient 2024. All Rights Reserved.