.webp)

Hall 9, A74
November 14-17 , 2022
Düsseldorf, Germany

The medical device industry has been seeing vital transformations in Quality Assurance and Regulatory Affairs in recent years. These changes include an increased focus on Clinical Evaluation, risk management, and post-market surveillance, harmonizing standards, and adopting digital technologies like AI and IoT. Medical Devices Enterprises are in a constant tussle to improve the safety and efficacy of medical devices and streamline regulatory processes.
Tracking and Management of Standards and Regulations
Interpretation and understanding of regulations and standards
Post Market Surveillance Activities & Adverse Event Reporting
Regulatory workflows and registration processes
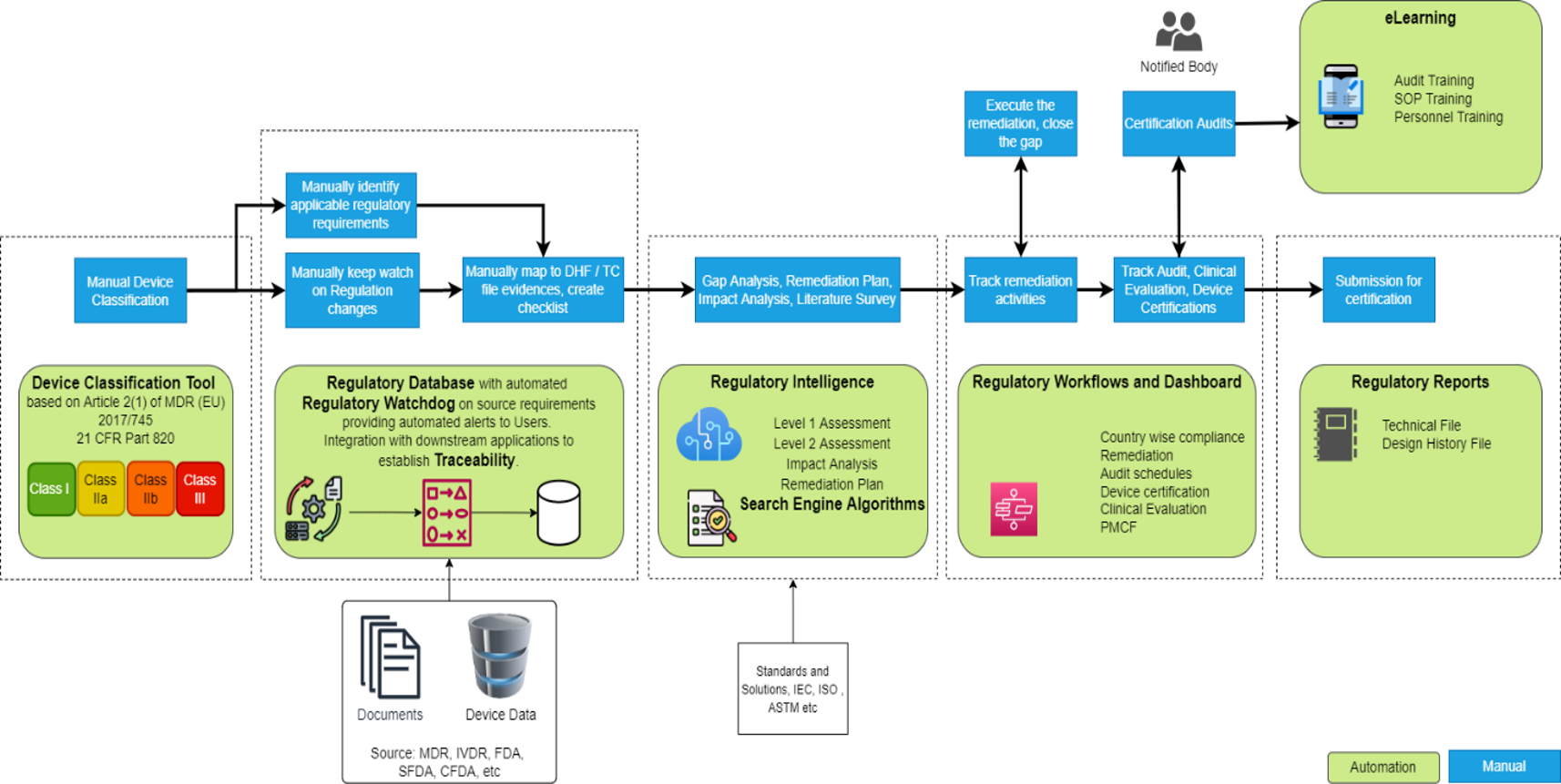
With improved efficiency, increased agility, and enhanced trust with customers, accelerating compliance gives an organizations a competitive advantage. CyARC is an intelligent solution designed to accelerate the regulatory compliance process. The solution is being demonstrated for Medical Device regulatory compliance. However, the same solution can be adapted for other regulatory industries like Aerospace, Rail, Mining, Nuclear etc.
Worldwide regulations scatter in various forms and places! Save time and efforts required to consolidate the data from various sources
Worldwide regulations scatter in various forms and places! Save time and efforts required to consolidate the data from various sources
Tool based on classification rules with automatic regulatory requirement map based on country registered
Tool based on classification rules with automatic regulatory requirement map based on country registered
An automatic Regulatory Watch to monitor changes in the regulations and create regulatory Alerts by subscribing to feeds
An automatic Regulatory Watch to monitor changes in the regulations and create regulatory Alerts by subscribing to feeds
Readymade EU MDR, and FDA compliance analysis templates with in-built regulatory intelligence based on device classification and device type
Readymade EU MDR, and FDA compliance analysis templates with in-built regulatory intelligence based on device classification and device type
Based on ML/Cognitive services to perform automatic gap analysis, plan remediation and avoid rework/mistakes in submissions
Based on ML/Cognitive services to perform automatic gap analysis, plan remediation and avoid rework/mistakes in submissions
Interactive Web-based User Interface with DASHBOARD and REPORTS for Regulatory Compliance Tracking and Planning
Interactive Web-based User Interface with DASHBOARD and REPORTS for Regulatory Compliance Tracking and Planning
Browse PubMed, Cochrane, Scholar, Google with smart SEARCH ENGINE ALGORITHM to optimize efforts on Literature Survey
Browse PubMed, Cochrane, Scholar, Google with smart SEARCH ENGINE ALGORITHM to optimize efforts on Literature Survey
e-Learning module for the training of standard operating procedures, regulations, changes
e-Learning module for the training of standard operating procedures, regulations, changes
Cyient is a leading consulting-led, industry-centric, global technology solutions company. We enable our customers to apply technology imaginatively across their value chain to solve problems that matter. Customers draw on Cyient’s expertise in engineering, manufacturing, and digital technology services and solutions to deliver and support their next-generation products that meet the highest standards of safety, reliability, and performance.
We are committed to designing tomorrow together with our globally prominent customers in the MedTech industry and providing a competitive advantage with proven integrated manufacturing solutions in compliance with ISO 13485:2016, REACH, and RoHS. Over the last 20+ years, we have enabled customers to deliver accessible and affordable high-quality healthcare solutions to millions of people worldwide.
To know more about our capabilities and experience in healthcare and life sciences, please visit https://www.cyient.com/healthcare-life-sciences
Cyient (Estd: 1991, NSE: CYIENT)delivers Intelligent Engineering solutions for Digital, Autonomous and Sustainable Future
© Cyient 2024. All Rights Reserved.