Intelligent Engineering
Our Intelligent Engineering solutions across products, plant and networks, combine our engineering expertise with advanced technologies to enable digital engineering & operations, develop autonomous products & platforms, and build sustainable energy and infrastructure
.png?width=774&height=812&name=Master%20final%201%20(1).png)
Global Regulatory Monitoring and Intelligence Platform for MDR Compliance
Empowering Medical Device Manufacturers with Cyient & Microsoft’s Agentic AI Solution CyientGlobal Regulatory Monitoring and Intelligence Platform for MDR Compliance
Empowering Medical Device Manufacturers with Cyient & Microsoft’s Agentic AI Solution
Abstract
AI-enabled automation is now essential for transforming regulatory complexity into operational efficiency and for ensuring sustained market readiness in a rapidly evolving compliance landscape.
The transition from MDD to MDR has intensified regulatory expectations, making documentation, re-certification, and continuous compliance significantly more demanding for medical device manufacturers. As timelines compress and manual processes struggle to keep pace, organizations require scalable, intelligent solutions that reduce effort without compromising regulatory rigor.
This whitepaper highlights how Cyient, powered by Microsoft technologies, leverages AI-driven automation to modernize MDR compliance. Intelligent checklists, automated gap assessments, remediation workflows, and audit-ready documentation accelerate certification, minimize errors, and improve regulatory confidence. By unifying data across PLM, ERP, QMS, and compliance systems, Cyient enables manufacturers to achieve faster, more accurate, and future-proof compliance.
Executive Summary
The regulatory compliance transition from the Medical Device Directive (MDD) to the Medical Device Regulation (MDR) has greatly increased the challenges and regulatory demands faced by medical device manufacturers. As regulatory requirements grow stricter, the burden of re-certifying legacy devices and ensuring ongoing compliance has become a complex, resource-intensive challenge. The sense of urgency is amplified as the MDR transition deadline approaches, especially for higher-risk device classes (Class 2B or Class 3) where the complexity of compliance is significantly greater and delays could jeopardize market continuity.
The MDR transition continues to rely on repetitive and rigorous manual activities, making it increasingly difficult for manufacturers to meet deadlines efficiently. It is therefore crucial to adopt smarter, intelligent and more efficient ways of working that simplify execution without replacing the critical regulatory decision-making that must remain with experts.
At Cyient, we understand the pressures faced by manufacturers in this new regulatory landscape. Our AI-powered automation solution transforms the MDR transition by replacing cumbersome, manual processes with intelligent, streamlined workflows. This approach not only accelerates certification timelines but also delivers continuous compliance and safeguards market continuity, while significantly reducing operational costs.
By harnessing advanced AI copilots, seamless data integration, and the scalable power of Microsoft technologies, we empower manufacturers to turn regulatory complexity into a strategic advantage. With our solution, you stay ahead of deadlines, minimize risk, and uphold the highest standards of quality and safety.
Access Whitepaper
Industry Challenges: Navigating the MDR Shift
The transition from MDD to MDR has created new challenges across the medical device industry, with stricter compliance standards, more comprehensive documentation requirements, and a greater emphasis on post-market surveillance and risk management.
Operational Strain
Manufacturers are facing bottlenecks due to limited notified body capacity, which creates delays in certification processes. Re-certification for legacy devices, even if unchanged, adds additional complexity.
Financial Pressure
MDR compliance costs are 2–3 times higher than MDD. Smaller manufacturers in particular are at risk of market exit due to resource constraints and rising costs.
Portfolio Rationalization
Many companies are discontinuing low-margin products and focusing on high-value, high-risk products with stronger clinical data. This shift increases the pressure on manufacturers to meet compliance deadlines efficiently in a timely manner.
Quality & Safety
MDR’s proactive post-market surveillance and clinical evaluation with risk management focus, drive improvements in product safety, but they also require robust data systems and rigorous data reporting which in turn calls for smart tools to manage the data effectively.
Cyient’s Solution: AI-Powered MDR Compliance:
Cyient’s AI-powered automation solution is designed to simplify the MDR transition by streamlining documentation, ensuring compliance, and accelerating certification. Our AI agents cover every aspect of the MDR process, from checklist generation to audit preparation.

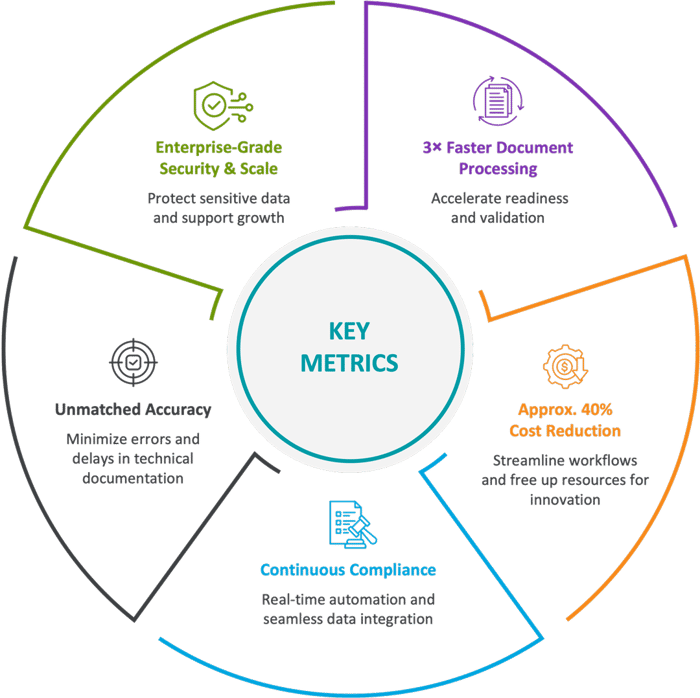
Driving Measurable Impact
Cyient’s AI-powered automation doesn’t just simplify the MDR transition; it transforms your entire compliance process, delivering tangible benefits across operational efficiency, accuracy, and business agility.


SHAPING THE FUTURE OF COMPLIANCE
MDR is reshaping the medical device industry, making compliance a strategic enabler of trust and innovation. Cyient’s AI-powered automation and Microsoft cloud technologies transform regulatory complexity into operational strength, delivering faster certification, sustained compliance, and measurable business impact.
NEXT STEPS
Ready to accelerate your MDR compliance journey?
Visit cyient.com or contact our team for a personalized consultation.
About the Authors
 |
Keerthi T |
Keerthi is a seasoned engineering leader with over three decades of cross-industry experience driving large-scale transformation across Aerospace, Automotive, Industrial, Healthcare, and Hi-Tech sectors. With 17+ years at a leading service provider as Vice President of Strategic Initiatives for Integrated Engineering Solutions (IES), he led global diversification programs and helped establish major engineering centers for organizations such as Bombardier Aerospace and GE Consumer Products; experience that shapes his deep understanding of compliance-driven engineering environments.
Keerthi is a certified PMP, Reliability Practitioner, and Six Sigma Master Black Belt (MBB), with a Mechanical Engineering degree from Bangalore Institute of Technology and an Executive Management Program from IIFT, New Delhi.
He also served as the Chief Design Engineer and architect behind India’s 108 Ambulance initiative, demonstrating his ability to translate stringent regulatory, safety, and quality requirements into scalable real-world impact.
 |
Abhishek Mishra |
Abhishek Mishra is a Quality, Regulatory, and R&D leader with 19+ years of global experience in the medical device industry, covering electromechanical systems, surgical robotics, and software-enabled devices. He has led functions across Quality, Regulatory Affairs, Clinical Evaluation, Systems Engineering, Supplier Quality, and Risk Management for major global OEMs.
He has driven ISO 13485 certifications, EU MDR and FDA 510(k) submissions, and global registrations across the US, EU, Canada, Middle East, and APAC. His expertise includes building scalable quality systems, leading cross-functional teams, and enabling regulatory excellence.
Previously at Medtronic, Abhishek managed quality assurance for multi-billion-dollar product lines, supporting V&V, manufacturing transfers, supplier qualification, and risk management.
He is well-versed in ISO 13485, 21 CFR 820, ISO 14971, IEC 60601, ISO 10993, and global regulatory frameworks. Abhishek holds a Bachelor’s degree in Mechanical Engineering from BIT Mesra and has worked across the USA, Singapore, and China.
About Cyient
Cyient (Estd: 1991, NSE: CYIENT) delivers intelligent engineering solutions across products, plants, and networks for over 300 global customers, including 30% of the top 100 global innovators. As a company, Cyient is committed to designing a culturally inclusive, socially responsible, and environmentally sustainable tomorrow together with our stakeholders.
For more information, please visit www.cyient.com